Consider the thermal decomposition
CH+3CH2CH∣CH3−+N(CH3)3OH−Δ−−→
Which of the following is the major product ?
A
But-1-ene
B
cis-But-2-ene
C
trans-But-2-ene
D
A mixture of (2) and (3)
Video Solution
Text Solution
The correct Answer is:A
Elimination proceeds by the removal of a β−H as proton. Any factor that affects its ease of removal will affect the rate of reaction. When alternatives β−H's are present, the one most readily removed as proton will decide the structure of the predominnating alkene product. Terminal βH's are more acidic than internal βH's. Therefore, but-1-ene is the major product.

We can predictd the major product using Hofmann's rule.
|
Topper's Solved these Questions
- ALKENESBook:R SHARMAChapter:ALKENESExercise:Follow-up-6
Explore 4 Videos
- ALKENESBook:R SHARMAChapter:ALKENESExercise:Follow-up-7
Explore 9 Videos
- ALKENESBook:R SHARMAChapter:ALKENESExercise:Follow-up-4
Explore 8 Videos
- ALKANESBook:R SHARMAChapter:ALKANESExercise:ARCHIVES
Explore 17 Videos
- ALKYNESBook:R SHARMAChapter:ALKYNESExercise:Archives
Explore 42 Videos
Similar Questions
Knowledge Check
In the reaction
CH3CH2CH2−CH3+∣N∣CH3−CH2CH3OH−Δ−−→
which of the following is formed in the largest amount?ACH2=CH−CH3BCH2=CH2CBoth (1) and (2) are formed in equimalor amountsDNo alkene is formedConsider the reaction
⎡⎢ ⎢ ⎢⎣CH3CH2CH2−CH3∣⊕(N)∣CH3−CH2CH3⎤⎥ ⎥ ⎥⎦OH−Heat−−→
Which of the following is formed in major amount ?ACH2=CH2BCH3CH=CH2CBoth (a) and (b) in equal amountDNone, as no reaction takes placeThennal decomposition of
⎡⎢ ⎢⎣CH3CH2−NH3∣N∣CH3−C∣CH3(CH3)2⎤⎥ ⎥⎦OH− givesACH3CH=CH2BCH2=CH2CCH3−CH3DCH3CH2CH3
CH3−CH3∣C∣CH3−CH2−OHconc.H2SO4−−−−−−−→ΔP
The product P is
View SolutionThe major product in the reaction is
CH3−C∣CH3H−CH2BrCH3O−−−−−→CH3OH
View SolutionThe major product in the reaction is
CH3−C∣CH3H−CH2BrCH3O−−−−−→CH3OH
View SolutionThermal decompositionn of ⎡⎢ ⎢⎣CH3−CH2−CH3∣N∣CH3−C∣CH3(CH3)2⎤⎥ ⎥⎦+OH− gives
View SolutionConsider the following reactions :
I :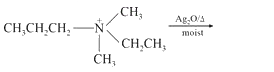
II. (CH3)2C∣OH−CH2−CHOΔ−−→
III. (CH3)2CHCH2−CH∣F−CH3CH3O−Na+−−−−−−−→Δ
IV. (CH3)2CH−C∣BrH−CH3(CH3)3CO−K+−−−−−−−−−−→
Which of these reaction(s) will not produce Hoffmann product ?
View Solution
