On strong heating, AgNO3 produces the gases
A
NO2 and NO
B
NO and O2
C
N2O and NO
D
NO2 and O2
Video Solution
Text Solution
The correct Answer is:D
Silver nitrate (Lunar caustic) is teachnically the mopst important of Ag compounds. It is prepared by dissolving metallic silver in dilute nitric acid:
3Ag+4HNO3 Δ−−→ 3AgNO3+NO+2H2O
(Dilute)
From solution it crystallizes as larger rhombic plates, mp. 482K. It is not hygroscopic and is very soluble is water. The solubility are susceptible to decomposition by light. Thus Ag NO3 is usually kept in amber-colored battles. When heated it decomposes in two stages: On heating above its melting points, it decomposes to silver nitrite and oxygen:
2AgNO3 723K−−→ 2AgNO2+O2
when heated at red heat, it further decomposes to metallic silver.
2AgNO3 980K−−→ 2Ag+2NO2+O2
Sliver nitratre is also decomposed by organic matter, such as glucose, paper, skin, and crok. It has also a caustic and destructive effect on organic tissues. In contact with oranic substances it blackens due to decomposition into metallic silver. Thus, it leaves black stains when comes in contant with skin and clothes. It produces burning sensation like the moon luna on skin and thus, clled Lunar caustic. Largequantites of AgNO3 are used in the production of light sensitive plates, films and papers. in the laboratories it is extensively used as a group reagent as a medicine in nervous diseases. A solution of AgNO3 is used in marking lines. It is also used in silvering of mirror.
|
Similar Questions
Knowledge Check
On strong heating Pb(NO3)2 gives:
APbOBNO2CO2DNO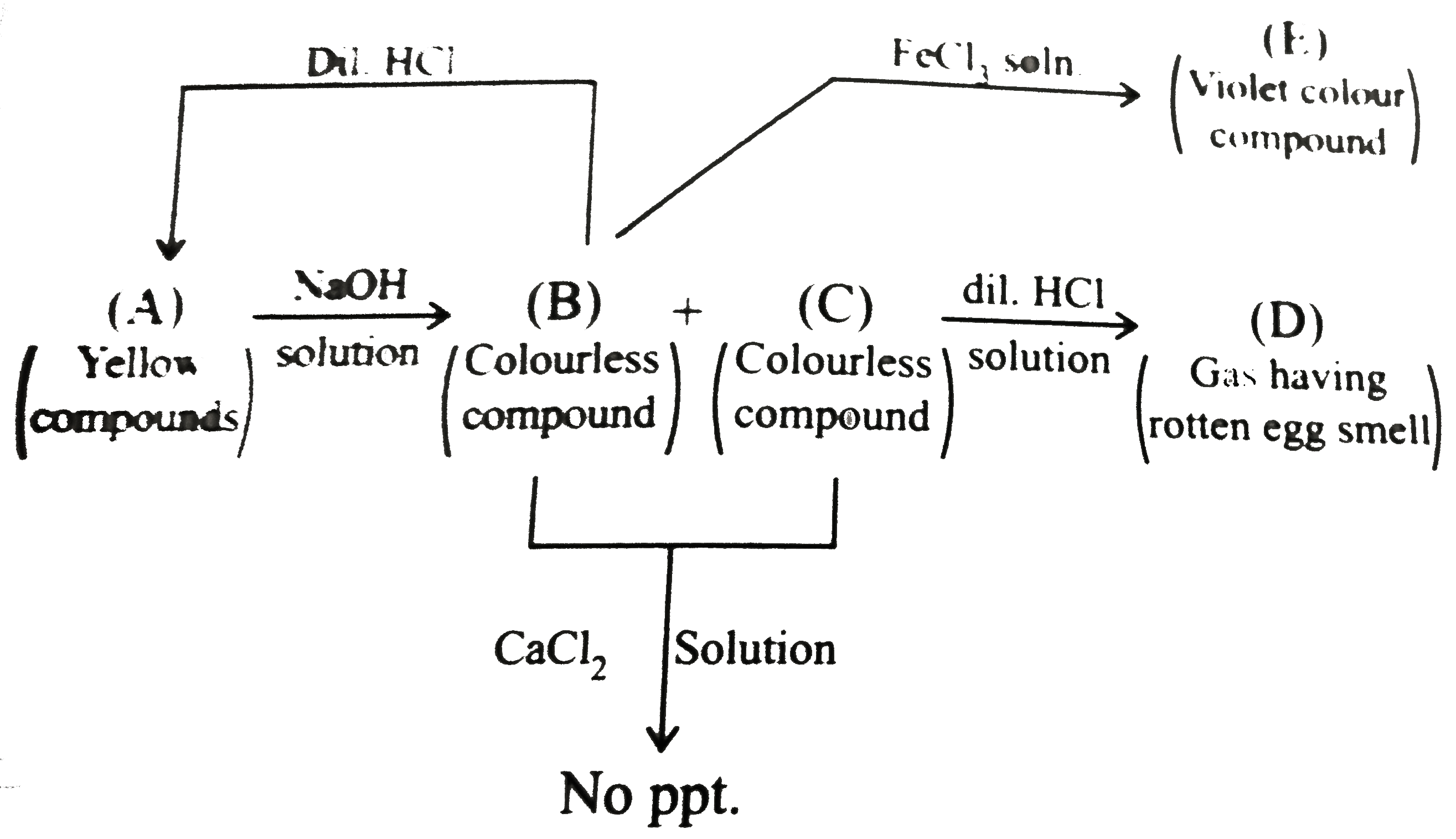
compound (B) on strong heating produces compound(s) which has/haveAChain structureBTetrahedral structureCBoth (a) and (b)DNone of these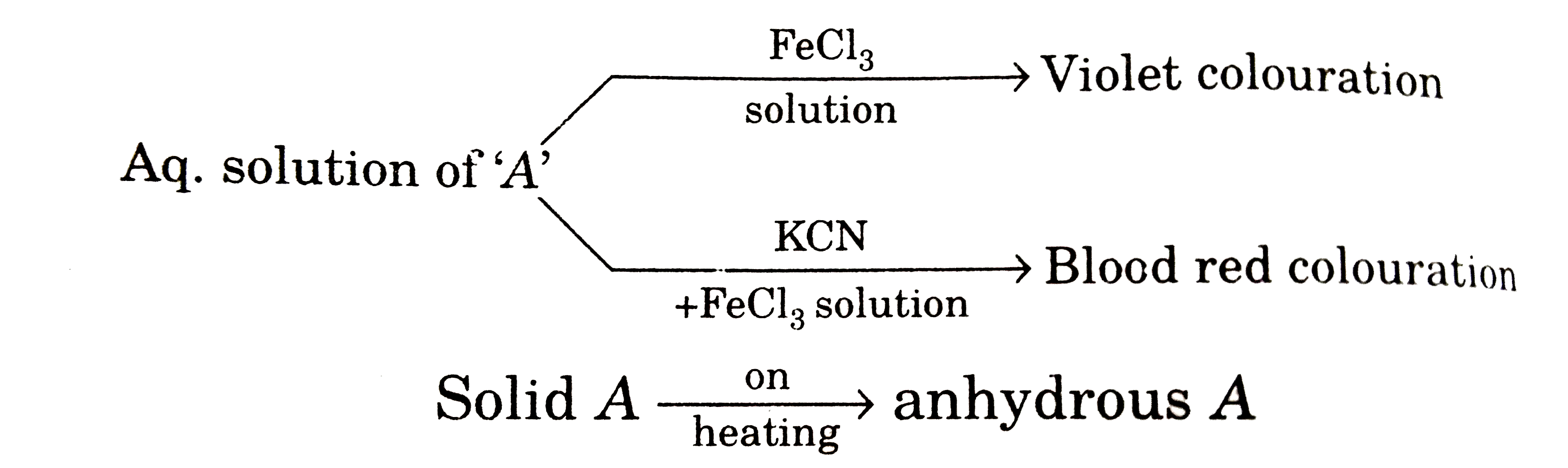
Solid Aon−−−→heating anhydrous A
A on strong heating produces compound(s) has/have:Achain structureBtetrahedral structureCboth (a) and (b)Dnone of the above
Calculate % yield of the reaction if 200gKHCO3 produces 22g of CO2 upon strong heating.
View SolutionCalculate % yield of the reaction if 1 Kg KHCO3 produces 110 g of CO2 upon strong heating.
View SolutionAqueous solution of CoCl3⋅6NH3 upon addition with AgNO3 produces 3 moles white precipitate . Primary and secondary valency of metal in this complex is
View SolutionWhen AgNO3 is strongly heated, the products formed are
View SolutionOn strong heating, ammonium acetate gives
View Solution
