In acidec medium, H2O2 changes Cr2O2−7 to CrO5 which has two (−O−O−) bonds. Oxidation state of Cr in CrO5 is
A
+5
B
+3
C
+6
D
−10
Video Solution
Text Solution
The correct Answer is:C
The structure of CrO5 is
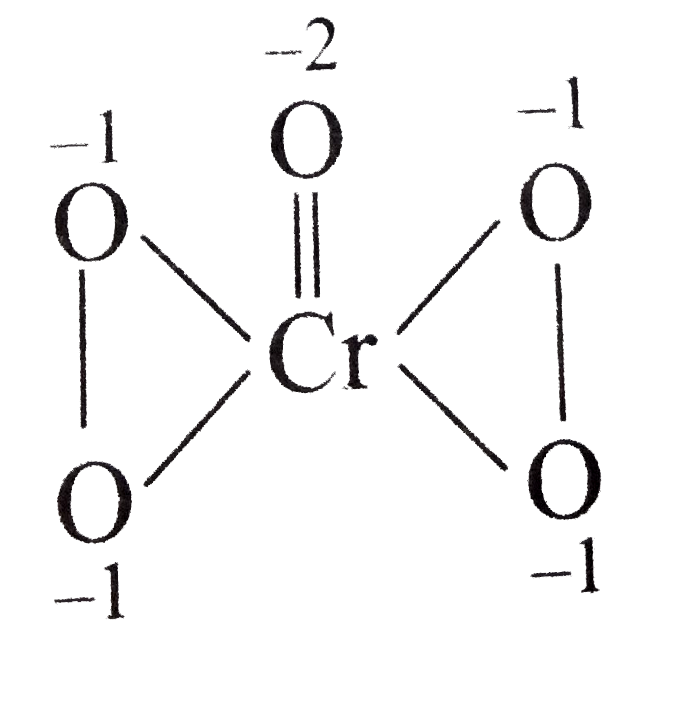
Out of five oxygen atoms, four are involved in peroxide linkages. Therefore, oxidation state of Cr is +6 .
|
Similar Questions
Knowledge Check
In acidic medium, H2O2 changes Cr2O2−7 to CrO5 which has two (-O-O-) bonds. Oxidation state of Cr in CrO5 is
A+5B+3C+6D−10In acidic medium, H2O2 changes Cr2O2−7 to CrO5 which has two (-O-O-) bonds. Oxidation state of Cr in CrO5 is
A+5B+3C+6D−10In acidic medium, H2O2 changes Cr2O−27 to CrO5 which has two (−O−O) bonds. Oxidation state of Cr in CrO5 is
A+3B+5C+6D−10
In acidic medium, H2O2 changes Cr2O−27 to CrO5 which has two (−O−O) bonds. Oxidation state of Cr in CrO5 is
View SolutionIn acidic medium, H2O2 changes Cr2O−27 to CrO5 which has two (−O−O) bonds. Oxidation state of Cr in CrO5 is
View SolutionThe oxidation state of Cr in CrO5 is:
View SolutionIn acidic medium, H2O2 changes Cr2O2−7 to CrO5 which has two (-O-O-) bonds. Oxidation state of Cr in CrO5 is
View SolutionIn acidic medium, H2O2 changes Cr2O2−7 to CrO5 which has two (-O-O-) bonds. Oxidation state of Cr in CrO5 is
View Solution

