Which of the following complexes will have the highest molar conductivity ?
CoCI3 . 3NH3
CoCI3 . 4NH3
CoCI3 . 5NH3
CoCI3 . 6NH3
The correct Answer is:D
The electrical conductivity of a solution of an ionic compound depends on:
(1) Concentration of solute
(2) Number of charges on the species which are formed on dissolution
Molar conductivity corresponds to a 1M solution and thus the concentration factor is removed. The total number of charges on the species formed when the complex dissolves in water can be deducted by complex dissolves in water can be deducted by comparison of its molar conductitity with that of known simple ionic materials.
[Co(NH3)6]C13→[Co(NH3)6]3++3C1−
[Co(NH3)5C1]C12→[Co(NH3)5C1]2++2C1−
[Co(NH3)4C12]C1→[Co(NH3)4C12]++C1−
[Co(NH3)3]C13→ No ionization
Total of 4 charges
CaC12→Ca2++2C1− mo1−1Scm−2
260.8
CoC13 . 5NH3 261.3
Total of 6 charges
LaC13→La3++3C1− 393.5
CoC13 . 6NH3 431.6
These conductivities sugguest the same structures for the cobaltammoniachlorine as do the results for werner's AgC1 experiments complexes AgC1 experiments.
Similar Questions
Combination between Pt(IV), NH3 and Cl− results in the formation of seven complexes. One of these complexes is [Pt(NH3)6]Cl4
(i) Write the formulae and IUPAC names of the other six complexes .
(ii) Which will have highest molar conductivity ?
(iii) Which of these is non-ionic ?
(iv) What is the the C.N of Pt in these complexes ?
View SolutionWhich one of the following solutions will have the highest molar conductance?
View Solution
Knowledge Check
which of the following compounds would have the highest molar conducitivity?
Adiamminedichloridoplatinum (II)Btetraamminedichloridocobalt (III)Cpotassium hexacynoferrate (II)Dhexaquachromium (III) chlorideWhich of the following complexes has the least molar conductivity in the solution?
ACoCl3.3NH3BCoCl3.4NH3CCoCl3.5NH3DCoCl3.6NH3Aqueous solution of which of the following complexes would exchibit the highest molar conductance?
A(a) K4[Fe(CN)6]B(b) K2[PtCl4]C(c) [Cr(NH3)3Cl3]D(d) [Co(NH3)6]Cl3
Which of the following option will be the limiting molar conductivity of CH3COOH if the limiting molar conductivity of CH3COONa is 91Scm2mol−1? Limiting molar conductivity for individual ions are given in the following table.
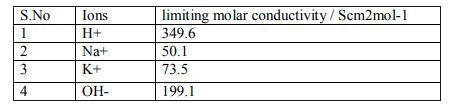
View SolutionWhich of the following has the highest molar conductivity in solution?
View SolutionWhich of the following complexes has lowest molar conductance ?
View SolutionWhich of the following complexes has lowest molar conductance ?
View SolutionWhich of the following solutions will have the highest specific conductance ?
View Solution
