Oxidation state of Fe in ferrocene is
A
0
B
+3
C
+2
D
+1
Video Solution
Text Solution
The correct Answer is:C
The oxidation number assigned does not necessarily relate to the actual charge on the metal. The convention used is that the organic moiety (C5H5) is usually assigned a charge of -1 In ferrocence, (η5−C5H5) Fe, iron has an oxidation state of +2.
|
Similar Questions
Knowledge Check
Ferrotes are formed in alkaline medium but they readily decopose to Fe2O3 and O2. Then oxidation state of iron in ferrates is
A+83B+38C+6D+2The oxidation state of Fe in brown ring complex [Fe(H2O)3NO]SO4 is :
A+1B+2C+3D+4Oxidation state of Fe in Fe3O4 is
A+2B+3C+8/3D+2/3
Oxidation state of Fe in FeO4 is:
View Solution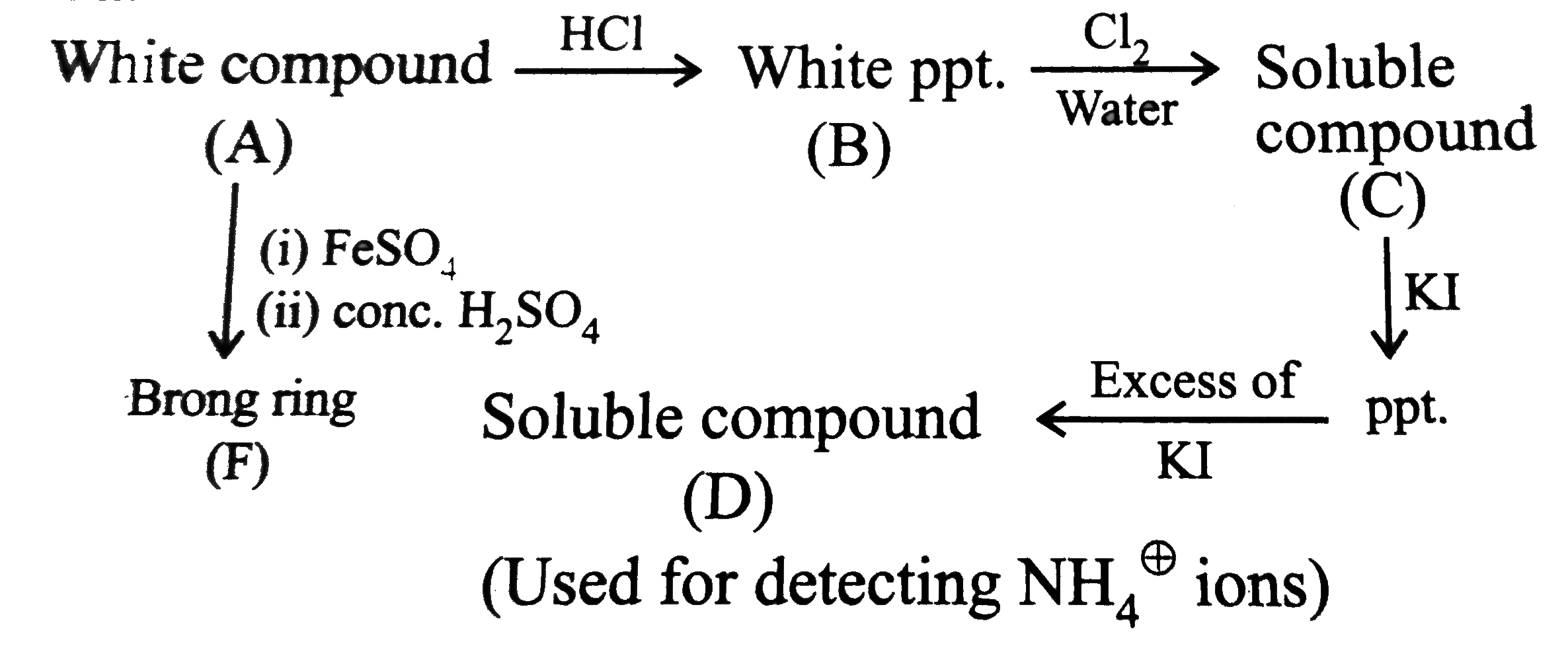
Oxidation state of Fe in compound (F) is
View SolutionSTATEMENT-1: Oxidation state of Fe in Fe(CO)5 is zero
and
STATEMENT-2: EAN of Fe in this complex is 36.
View SolutionAssertion: Oxidation state of Fe in Fe(CO)5 is zero.
Reason: Synergic bonding takes place in this metal carbonyl complex.
View SolutionAssertion: Oxidation state of Fe in Fe(CO)5 is zero.
Reason: Synergic bonding takes place in this metal carbonyl complex.
View Solution
