The number of isomers for the compound with moelecular formula C2BrCIFI is
A
5
B
4
C
6
D
3
Video Solution
Text Solution
The correct Answer is:C
First calculate the degree of unsaturation or double bond equivalents (DBEs):
DBE=∑n(v−2)2+
=2(4−2)+1(1−2)+1(1−2)+1(1−2)+1(1−2)2+1
=02+1=1
This implies the presence of one C=C or a cyclic structure, but on cyclie structure is possible as there are just two carbon atoms.
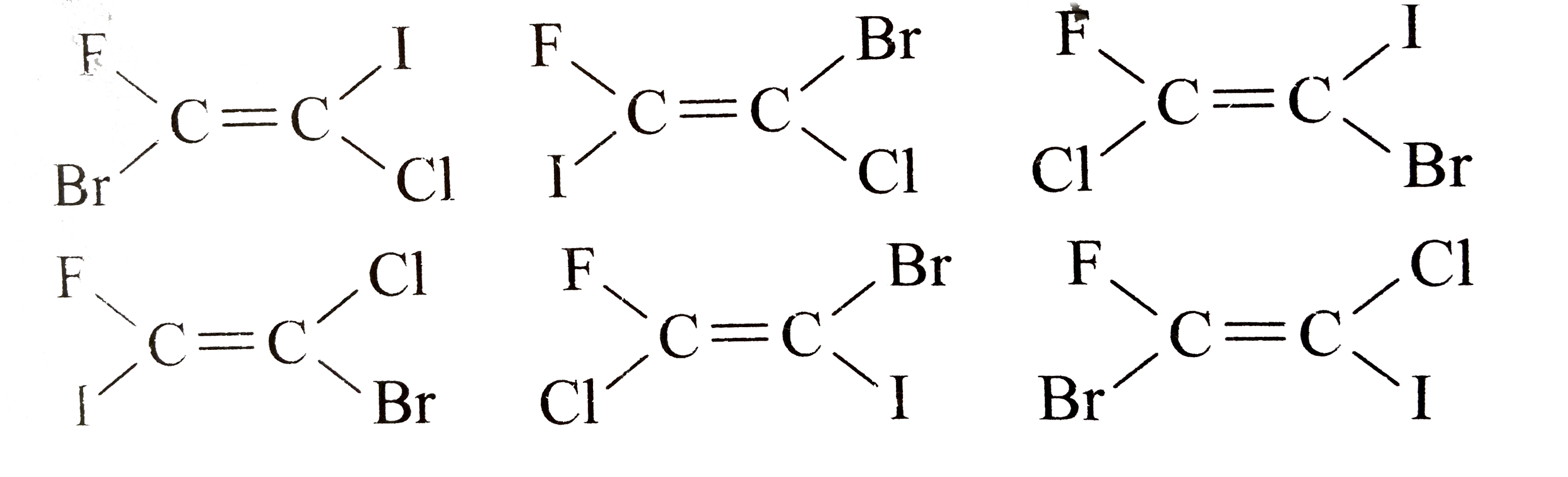
|
Similar Questions
Knowledge Check
The number of isomers for the compound with molecular formula C2BrCIFI is:
A3B4C5D6The number of isomers for the compound with molecular formula C2BrClFI is
A3B4C5D6The number of isomers for the compound with molecular formula C2BrClFI is:
A3B4C5D6
The number of isomers for the compounds with molecular formula C2BrClFI is:
View SolutionThe number of isomers for the compound with molecular formula C2BrClFl is
View SolutionThe number of isomers for the compound with molecular formula C2BrClFl is
View SolutionThe number of isomers for the compound with molecular formula C2BrClFI is
View SolutionThe number of isomers for the compound with molecular formula C2BrClFI is :
View Solution
