In the reaction
CH3CHO+HCN→CH3CH(OH)CNH2O−−→CH3CH(OH)COOH
an asymmertic cabron is generated. The acid obtained would be
A
D−isomer
B
L−isomer
C
50%D and 50%L isomer
D
20%D and 80%L isomer
Video Solution
Text Solution
The correct Answer is:C
Synthesis fo a chiral compound, CH3CH(OH)CN, from achiral reactants, CH3CHO and CN−, always yields the racemic modification.
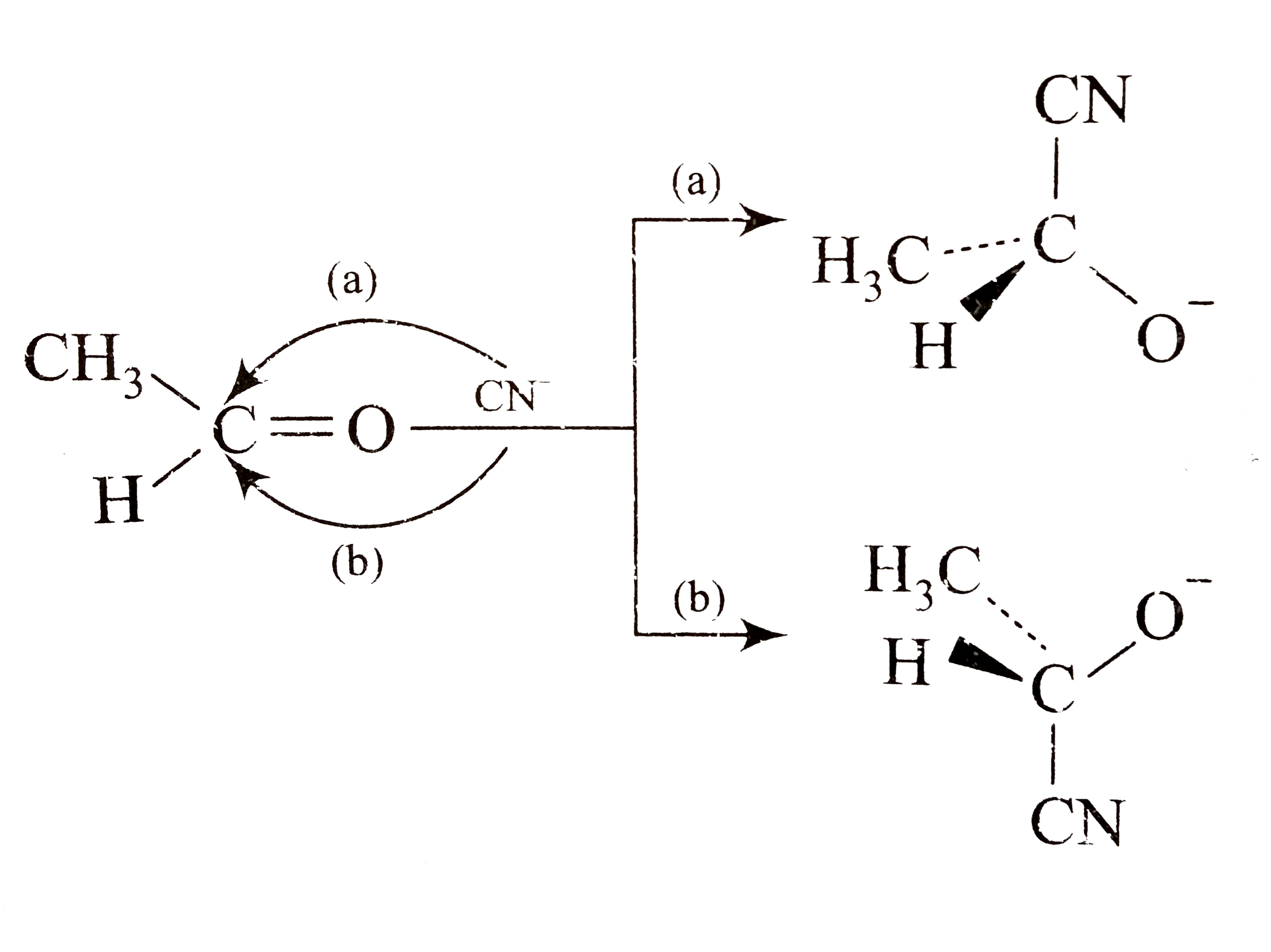
The flat aldehyde is said to have enatiopic faces because attachement of a fiurth ligands to one or the other face gives one or the other enantiomer. These attacks occur with equal probability, resulting in equal amounts of D and L (a racemic mixture).
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
CH3CHO+HCN→CH3CH(OH)CNH+/H2O−−−−−→CH3CH(OH)COOH an asymmetric centre is generated. The acid obtained would be
AD-isomerBL-isomerC50% D + 50%LD20% D + 80% L.In the reaction
CH3CHO+HCN→CH3CH(OH)CNH.OH−−−→CH3CH(OH)COOH
an asymmetric centre is generated. The acid obtained would beA50%D+50% L-isomerB20%D+80% L-isomerCD-isomerDL-isomerIn the reaction
CH3CHO+HCN→CH3CH(OH)CNH.OH−−−→CH3CH(OH)COOH
an asymmetric centre is generated. The acid obtained would beA50%D+50% L-isomerB20%D+80% L-isomerCD-isomerDL-isomer
In the reaction
CH3CHO+HCN→CH3CH(OH)CNH2O−−→CH3CH(OH)COOH
an asymmertic cabron is generated. The acid obtained would be
View SolutionIn the reaction : CH3CHO+HCN→CH3CH(OH)CNH.OH−−−→CH3CH(OH)COOH an asymmetric centre is generated. This acid obtained would be :
View SolutionThe reaction
CH3CHO+CH3CHODil. alkali−−−−−→CH3CH(OH)CH2CHO is known as
View SolutionThe reaction, (CH3)3C−IH2O−−→(CH3)3C−OH is
View SolutionCH3CHO+NH2OH−H2O−−−→CH3CH=NOH
The reaction is carried at :
View Solution
