Most amino acids? (AA's) have the configurations
A
R and L
B
R and D
C
S and L
D
S and D
Video Solution
Text Solution
The correct Answer is:C
Stereochemical studies of naturally occurring amino acids have shown that all have the same configuration about the carbon atom carrying the alpha - amino group, and that this configuration is the same as that in L−(−)− glyceraldehyde. Since the group R nearly always happens to have a lower [chains - Ingold - Prelog] priority than COOH, most of these have the S configuration
There are two amino acids that, although, L, like the other, happen to have the R configuration. They are (−) -cysteine and (−)− cystine. In all the amino acids. H has the lowest priority and +NH3 has the highest. In ost amino acids, COO− has priority over R because of the O once removed from the chiral center. But in cysteine and cystine, R contains S once removed from the chiral cener and this gives R priority over COO− (Because S has a greater atomic number than O, the CH2SH Group of cysteine has priority over the COO−). The reversal of priority of these groups ruesults in a change in specification from S and R.
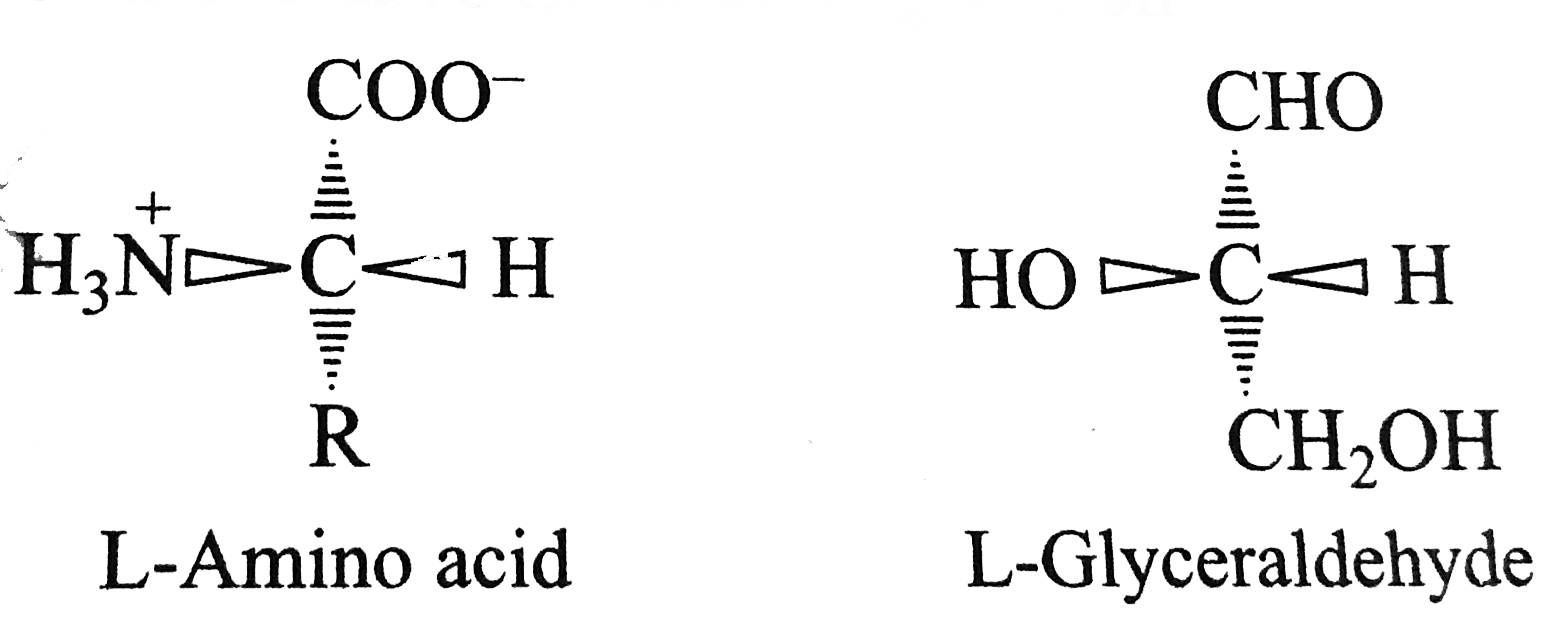
|
Similar Questions
Knowledge Check
An amino acid is
AReninBPepsinCCystineDProline.Amino acids are
AnonpolarBamphotericCacidicDbasicAmino acids are
AacidicBbasicCamphotericDneutral
Amino acids are:
View SolutionMost simple amino acid is
View SolutionMost simple amino acid is
View SolutionAssertion (A) All naturally occurring α-aminoacids except glycine are optically active.
Reason (R) Most naturally occurring amino acids have L-configuration.
View SolutionAssertion: All naturally occuring α-amino acids are optically active
Reason: Most naturally occuring amino acids have D-configuration.
View Solution
