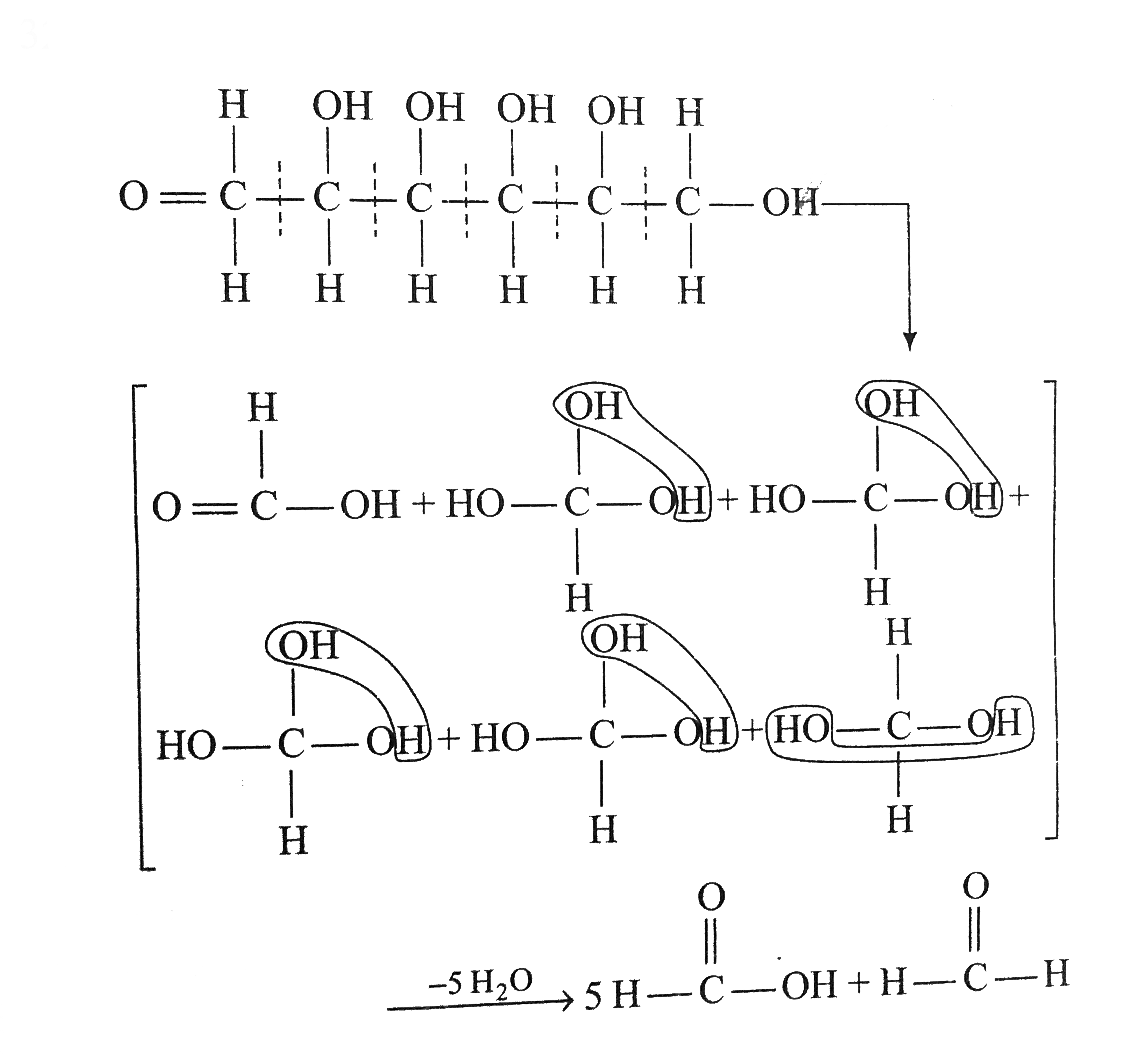
When glucose is treated with an execss of HIO4, the products formed are
A
six moles of HCOOH
B
one mole of oxalic acid (HOOC−COOH) three moles of HCOOH and one mole of HCHO
C
five mole of HCOOH and one mole of HCHO
D
one moles of glyoxal (OCH−CHO) one mole of HCHO and three moles of HCOOH
Video Solution
Text Solution
The correct Answer is:C
Thus, 5 moles of periodic acid (HIO4) are consumed per mole of the glucose as five C−C bonds have been cleaved.
|
Similar Questions
Knowledge Check
If sucrose iss treated with conc. H2SO4, the product formed is
ACO2BCCCODH2OIf sucrose is treated with conc. H2SO4, the product formed is
ACO2BCCCODH2OWhen SnCl2 is treated with HgCl2, the products formed are
ASn+HgCl4BSn+Cl2+Hg2Cl2CSnCl4 and Hg2Cl2Dnone of these
(1) What product would be formed when D-Glucose is treated with HIO4 ?
(2) D-Threose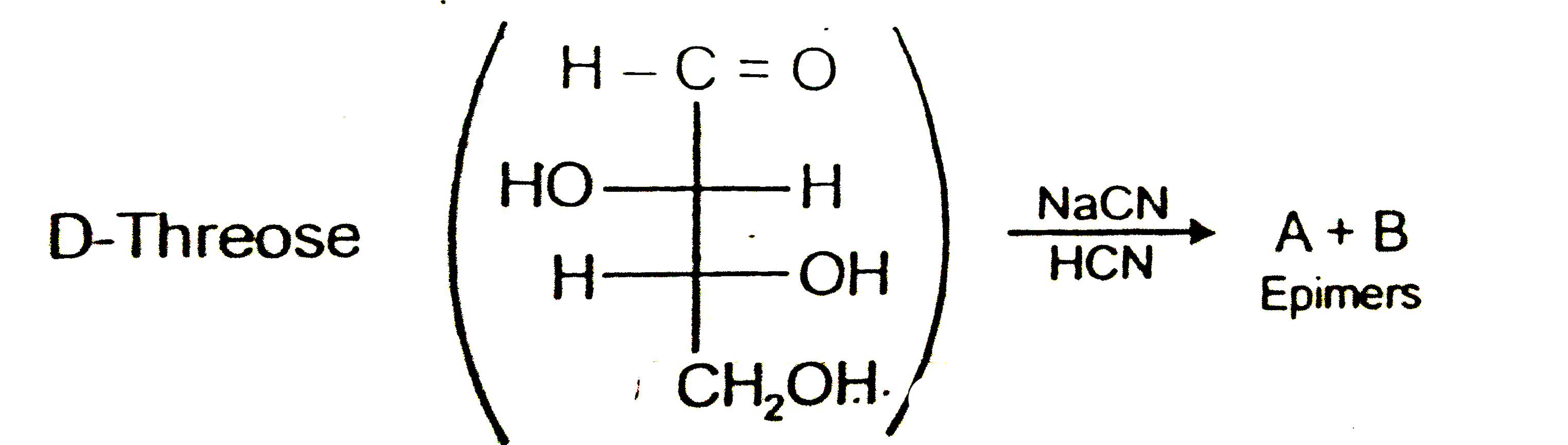
Why epimers formed are in unequal amounts?
View SolutionOne mole of benzene is treated with an excess of ozone and the product formed is then treated with zinc dust and water. The final product formed is.
View SolutionWhen on excess of chlorine is treated with ammonia ,the products formed are
View SolutionWhich 2-butyne is treated with dil .H2SO4/HgSO_(4), the product formed is
View SolutionWhen phenol is treated with excess of bromine water ,the product formed would be
View Solution

