In the reaction
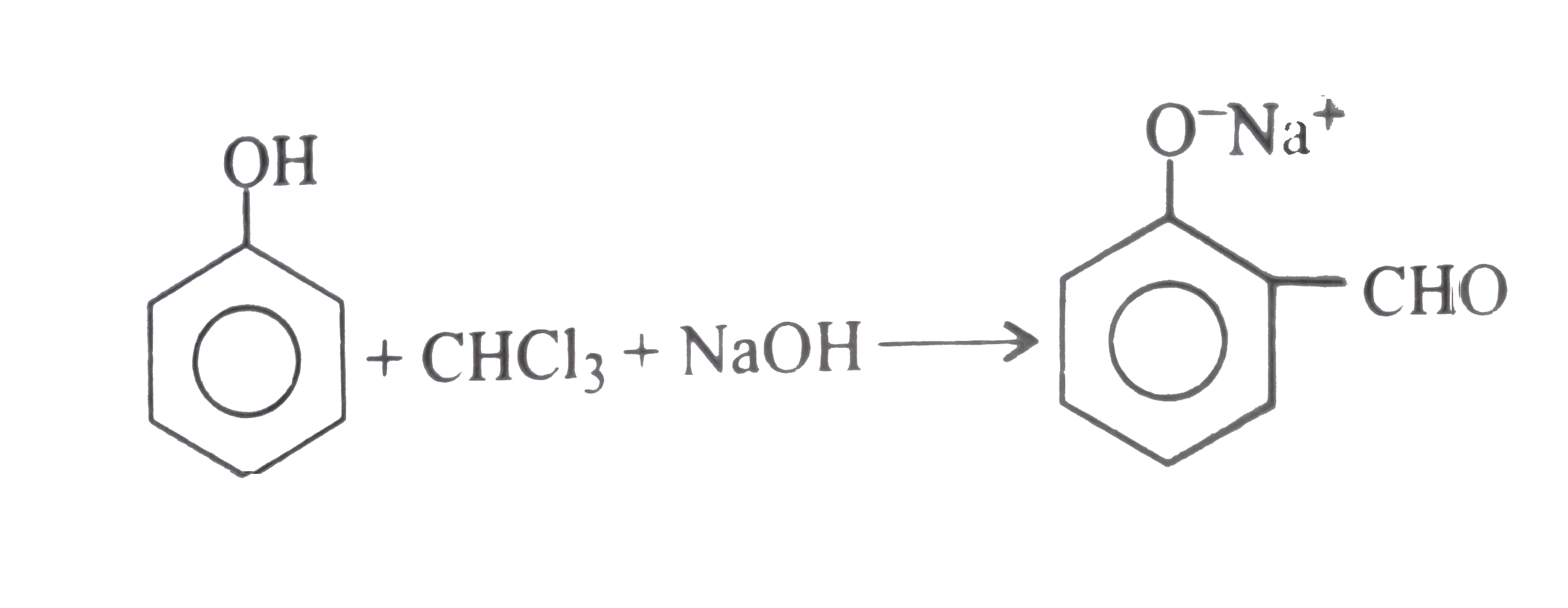 the electrophile involved is
the electrophile involved is
dichloromethyl cation ⊕CHCl2
formyl cation ⊕CHO
dichloromethyl anion ⊕CHCl2
dichlorocarbene (:CCl2)
The correct Answer is:D
Here the electrophile is dichlorocarbene (:CCl2) which is formed as follows:
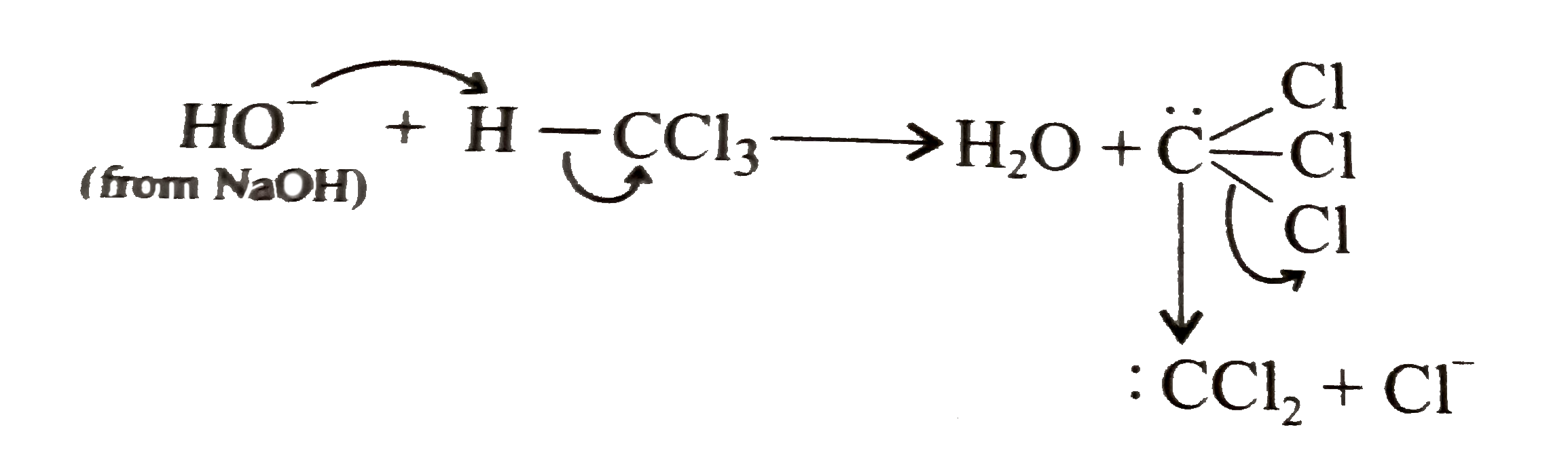
Similar Questions
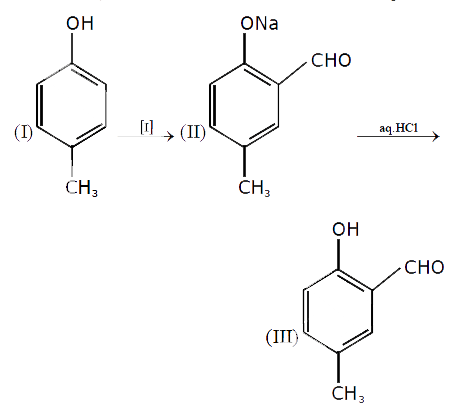
Reimer -Tiemann reaction introduces an aldehyde group on to the aromatic ring of phenol, ortho to the hydroxyl group. This reaction involves electrophilic aromatic substitution. This is a general method for the synthesis of substituted salicyladehydes as depicted below.
The electrophile in this reaction is
View SolutionIn a Friedel-Crafts reaction, the electrophile is:
View Solution
Knowledge Check
Consider the following reaction,
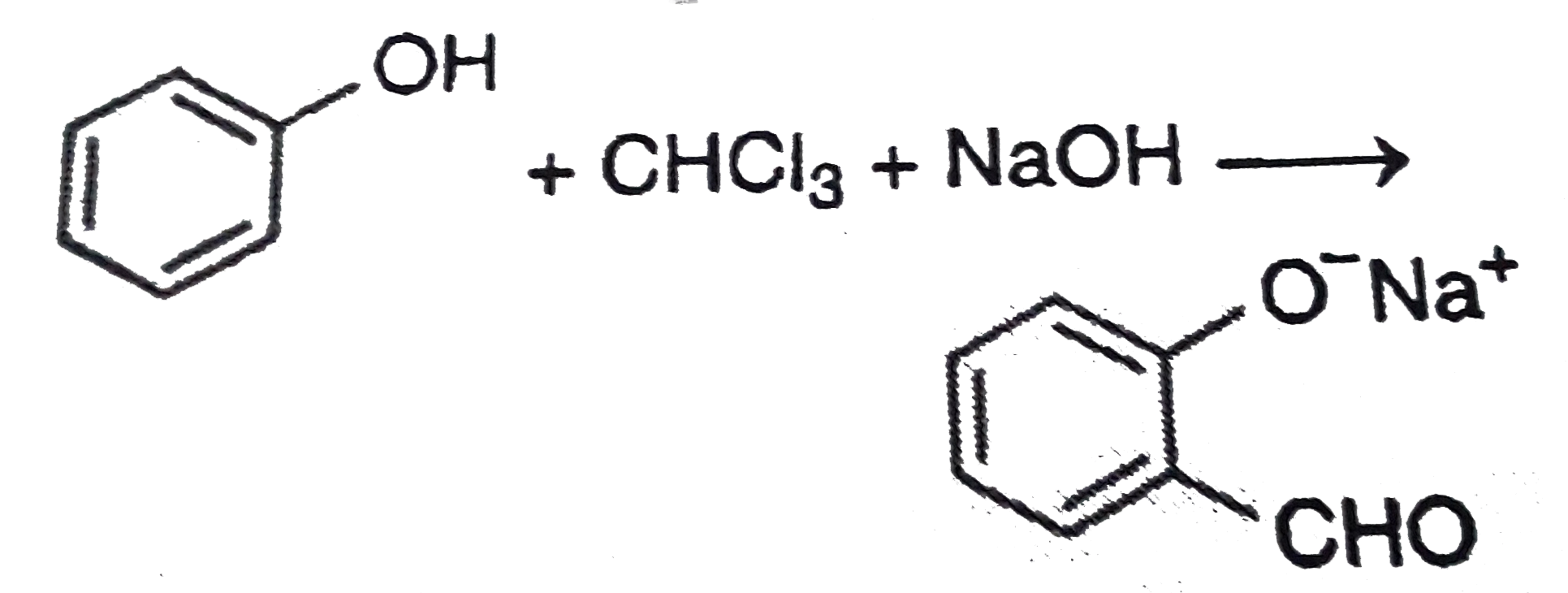
The electrophile involved in the above reaction isAdichloromethyl cation, +CHCl2Bformyl cation, +CHOCtrichloromethyl anion, ¯¯¯CCl3Ddichlorocarbene, : CCl2In the sulphonation of benzene, the electrophile involved is:
AHSO−4BSO3CSO2DSO2−4In Friedel-Carfts acylation reaction the electrophile is :
AR⊕BR−⊕C=OCR−⋅C=ODR- underset(Cl)underset(|)overset(o+)C-O - overset+(ө)AlCl_(3)
In the Friedel-Crafts acylation reaction, the electrophile is
View SolutionWhat is the first step in the reaction of an electrophile (E)) with benzene?
View SolutionReimer Tiemann reaction is an electrophillic substitution reaction in which the electrophile is
View SolutionC6H5OH+CHCl3+NaOH→ salicyladehyde The electrophile involved in the above reaction is.
View SolutionReimer-Tiemann reaction introduces an aldehyde group, on to the aromatic ring of phenol, ortho to the hydroxyl group. This reaction involves electrophilic aromatic substitution. This is a general method for the synthesis of substituted salicyladehydes as depicted below.
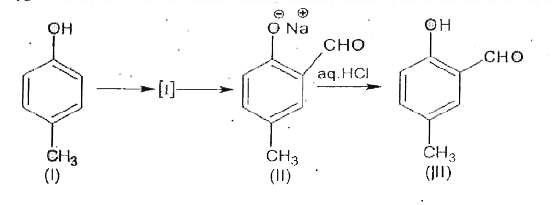
The electrophile in this reaction is
View Solution

