For gas at a temperature T the root-mean-square speed vrms, the most probable speed vmp, and the average speed vav obey the relationship
vav>vrms>vmp
vrms>vav>vmp
vmp>vav>vrms
vmp>vrms>vav
The correct Answer is:B
Step by step video, text & image solution for For gas at a temperature T the root-mean-square speed v_(rms), the most probable speed v_(mp), and the average speed v_(av) obey the relationship by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Similar Questions
माना कि ¯v,vrms तथा vp क्रमशः एकपरमाणुक आदर्श गैस में परमताप T पर अणुओं की औसत चाल (Mean speed), वर्ग - मध्य - मूल चाल (Root - mean - square speed) तथा सर्वाधिक प्रायिक चाल (Most probable speed) है | एक अणु का द्रव्यमान m है, तब
View SolutionVav,VrmsandVmp are average speed, root mean square speed and most probable speed of the gas respectively at constant temp. T. Molar mass of gas is 'm'. Then
View SolutionThe root mean square velocity, V_(rms) ,the average velocity v_(av) and the most probable velocity, v_(mp) of the molecules of the gas are in the order
View SolutionIdentify the correct labels of A, B and C in the following graph from the options given below:
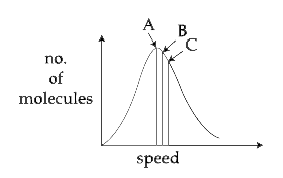
Root mean square speed (Vrms, most probable speed (Vmp, Average speed (Vav)
View SolutionFor a gas at a temperature T the Root mean square velocity vrms ,the most probable speed vmp,and the average speed aav obey the relationship
View SolutionFor gas at a temperature T the root-mean-square speed v_(rms) , the most probable speed v_(mp) , and the average speed v_(av) obey the relationship
View SolutionSuppose a container is evacuated to leave just one molecule of a gas in it. Let vmp and vav represent the most probable speed and the average speed of the gas, then
View SolutionIf Maxwell distribution is valid and v_(p) . denotes the most probable speed, v the average speed and v_("rms") the root-mean-square speed, then
View Solution

