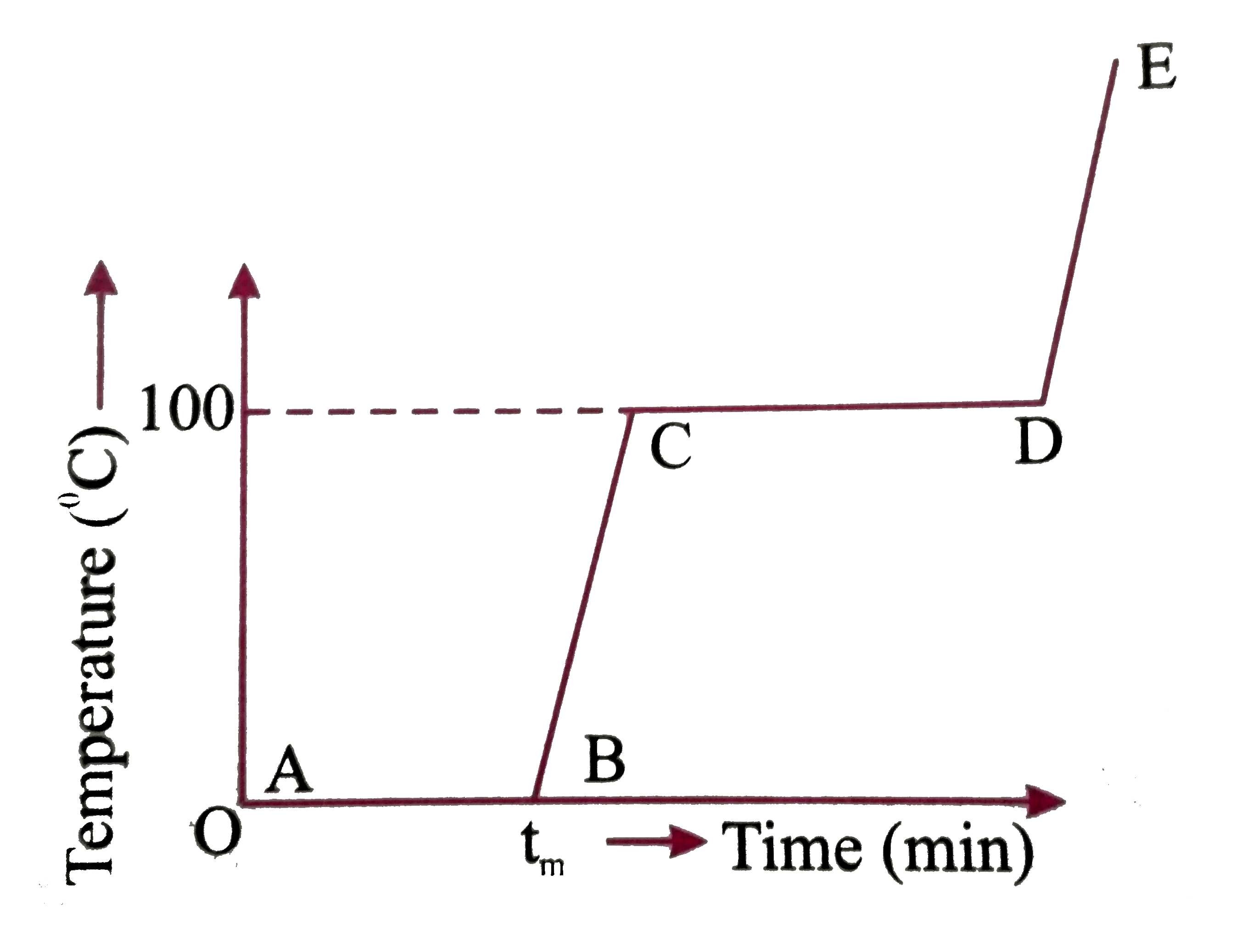
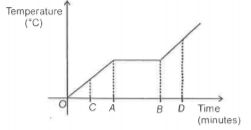
Refer to the plot of temperature versus time (figure) showing the changes in the state if ice on heating (not to scale). Which of the following is correct ?
 .
.
The region AB represents ice and water in thermal equilibrium.
At B water starts boiling
At C all the water gets converted into steam
C to D represents water and steam in equilibrium at boiling point.
The correct Answer is:A, D
During plase change process, temperature of the system remains constant.
Similar Questions
Knowledge Check
Refer to the plot of temperature versus time, showing the changes in the state of ice on heating (not to scale).
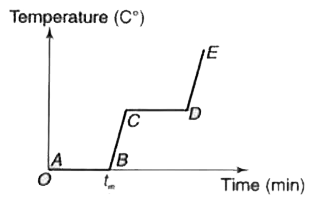
which of the following is correct ?AThe region AB represents steam and water in thermal equilibriumBAt B water starts boilingCAt C all the water gets converted into steamDC and D represent water and steam in equilibrium at boiling pointRefer to the plot of temperature versus time showing the changes in the state of ice on heating (not to scale).
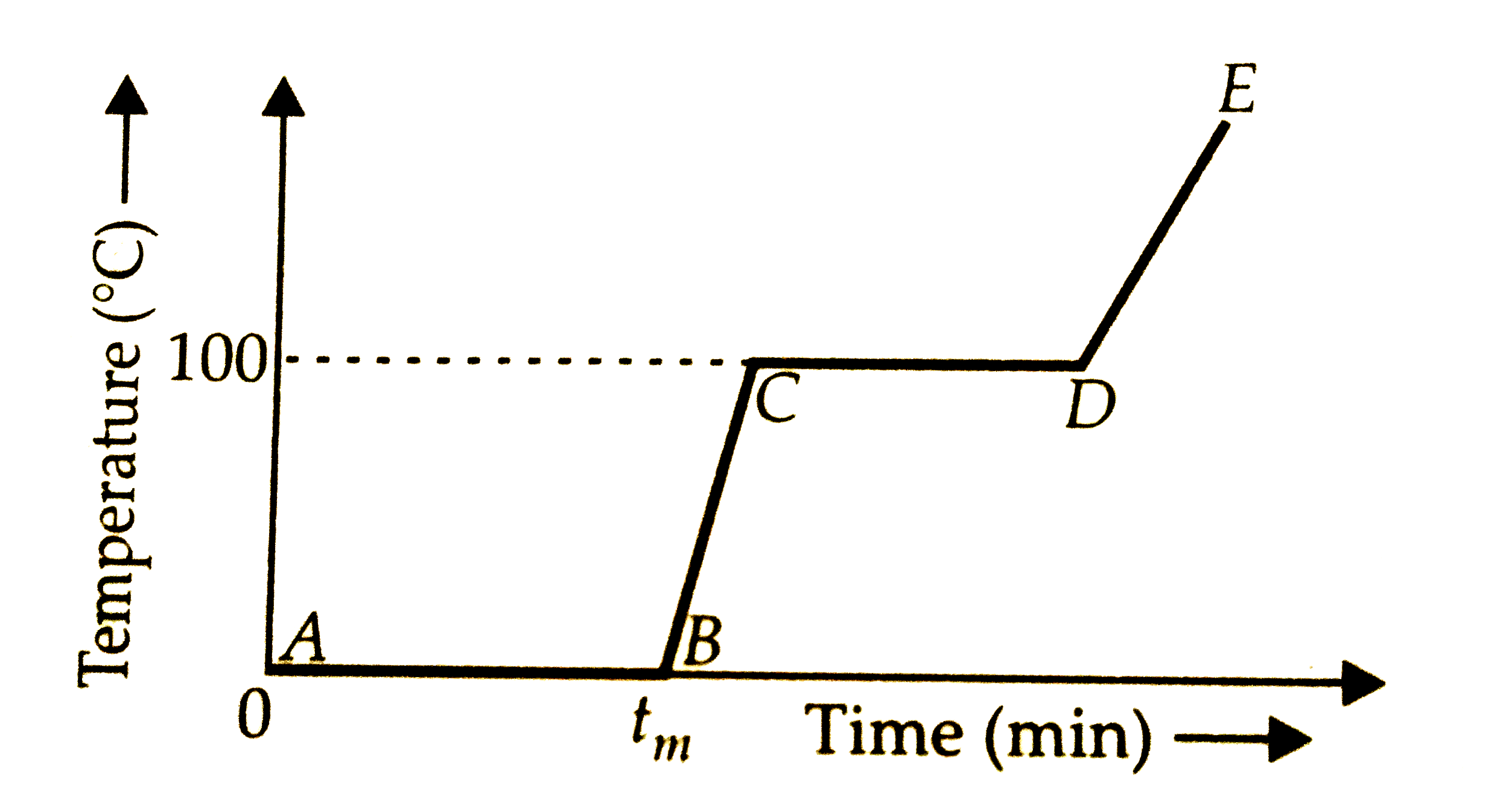
Which of the following is correct ?AAB represents ice and water are not in the thermal equilibriumBAt B water starts boiling.CAt C all the water gets converted into steam.DCD represents water and steam in equilibrium at boiling point.For gaseous state at constant temperature which of the following plot is correct?
A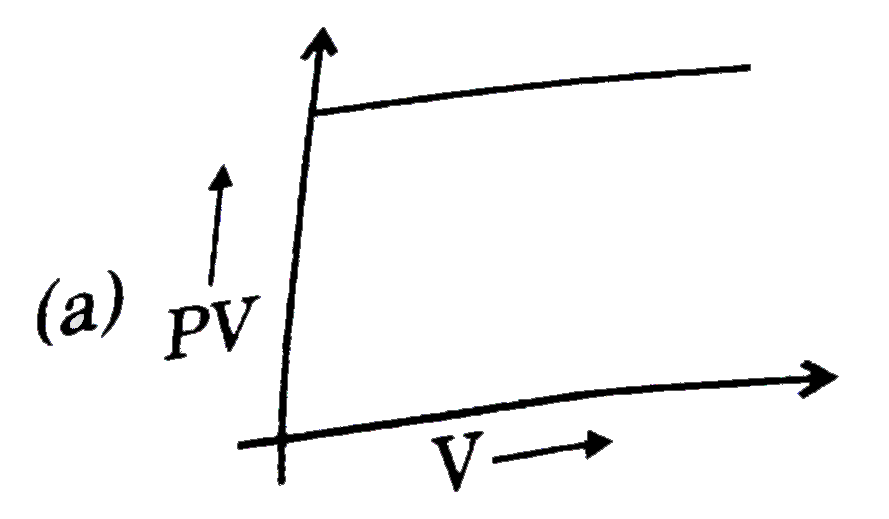 B
B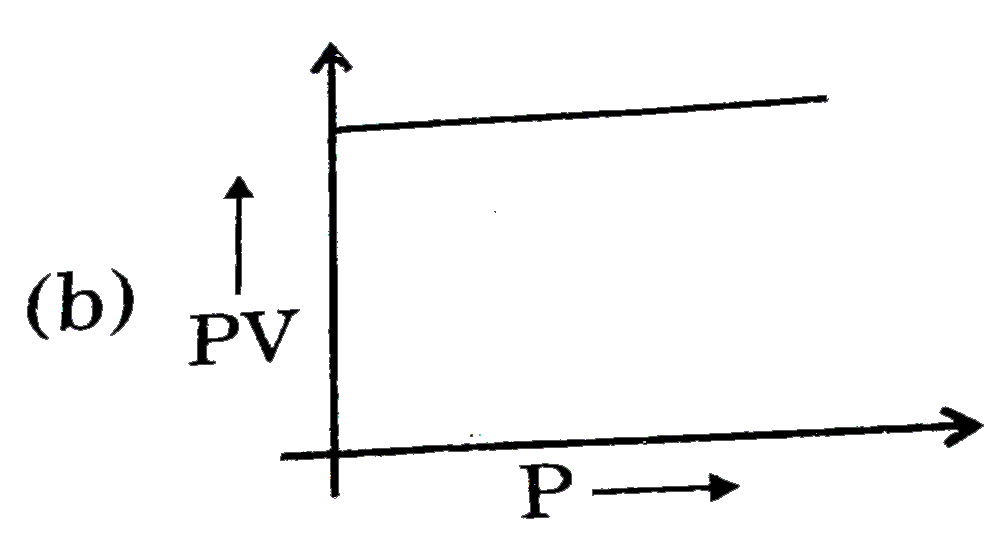 C
C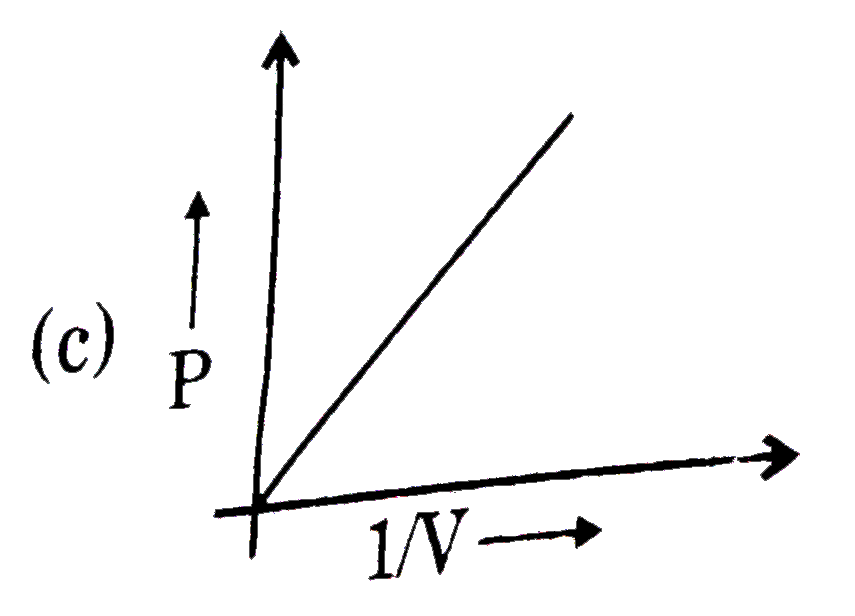 D
D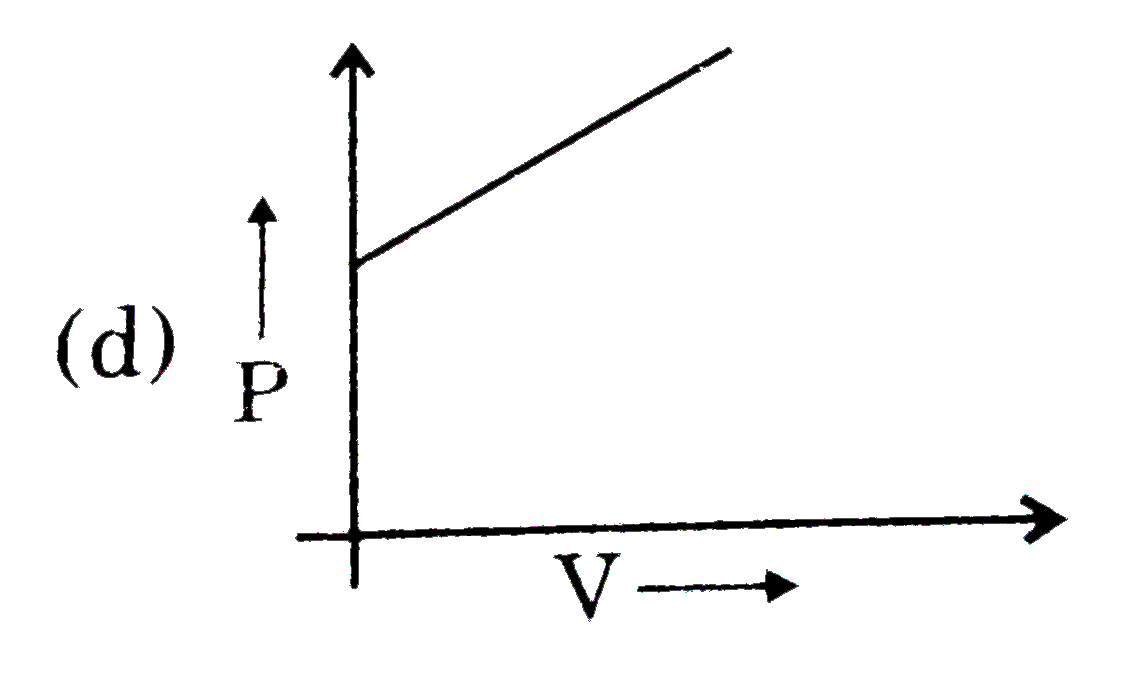
For the following temperature-time graph, in which region the state of material changes
View SolutionRefer to the given figure and answer the following questions
Which of the following statement is correct?
View SolutionA lump of ice floats in water as shown in the figure .

Which of the following statements is correct ?
View SolutionThe figure shows a plot of photo current versus anode potential for a photosensitive surface for three different radiations. Which one of the following is a correct statement ?
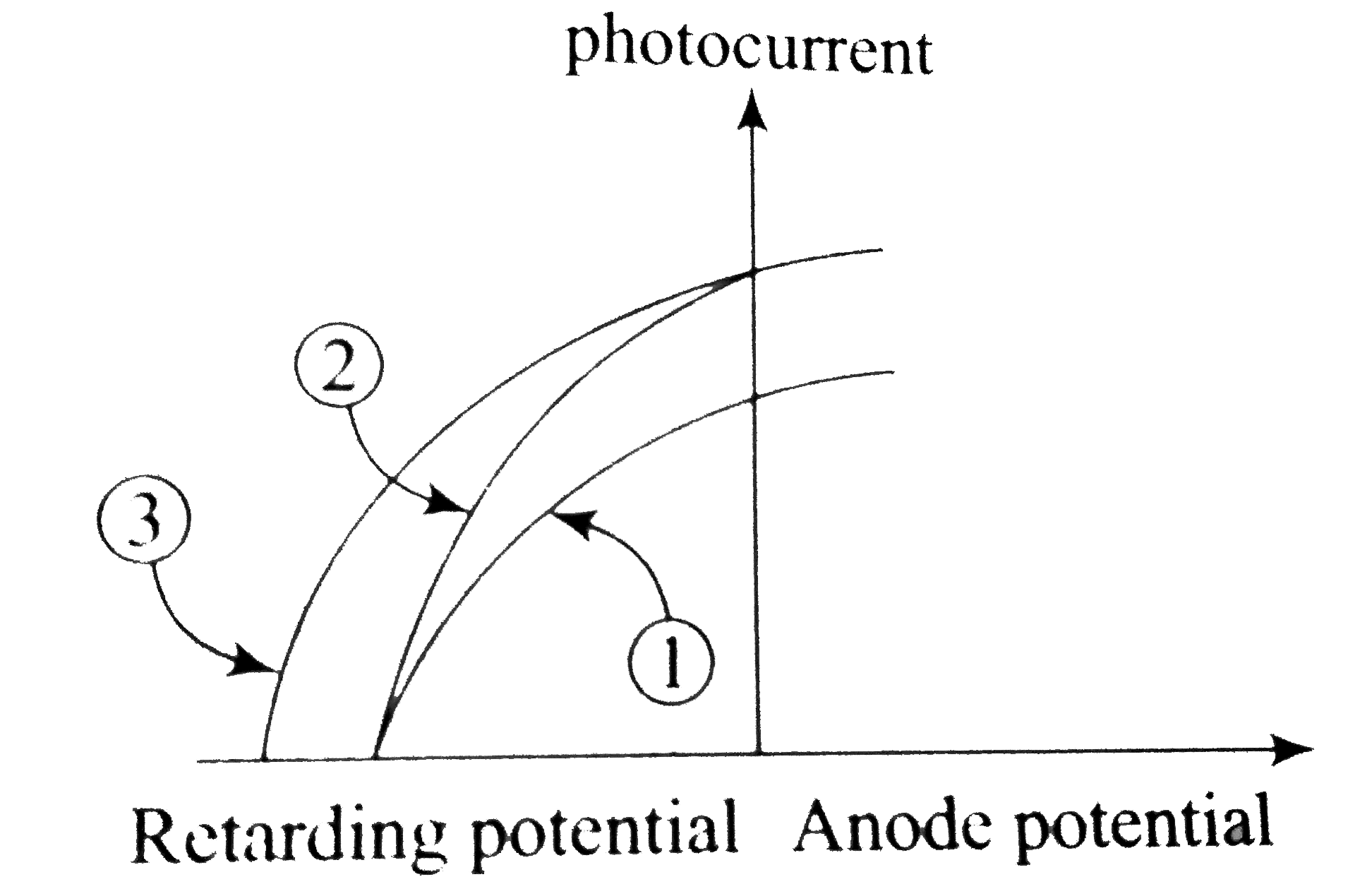
View SolutionThe temperature change versus heat supplied curve is given for 1 kg of a solid block. Then, which of the following statement is/are correct ?
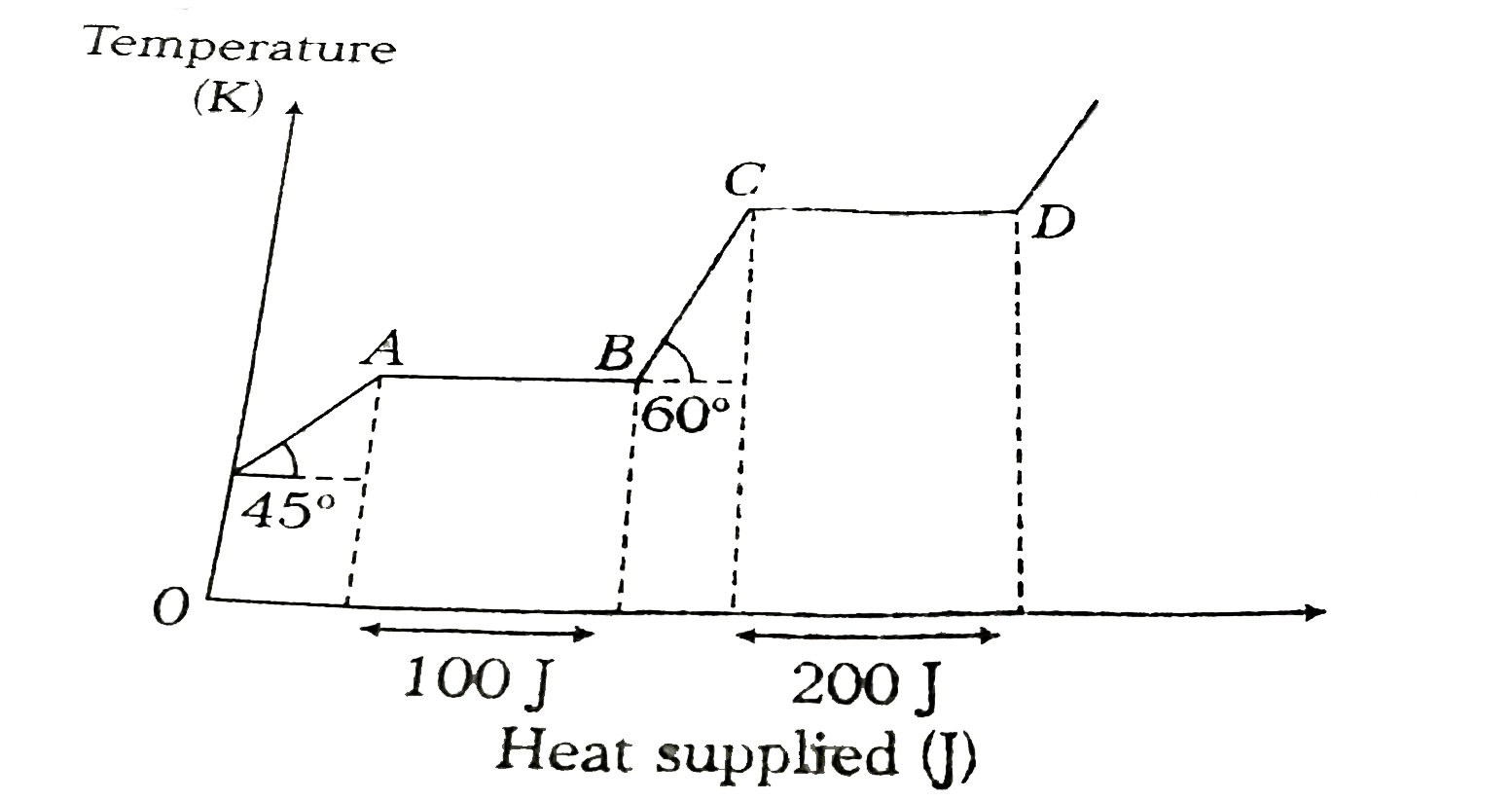
View Solution

