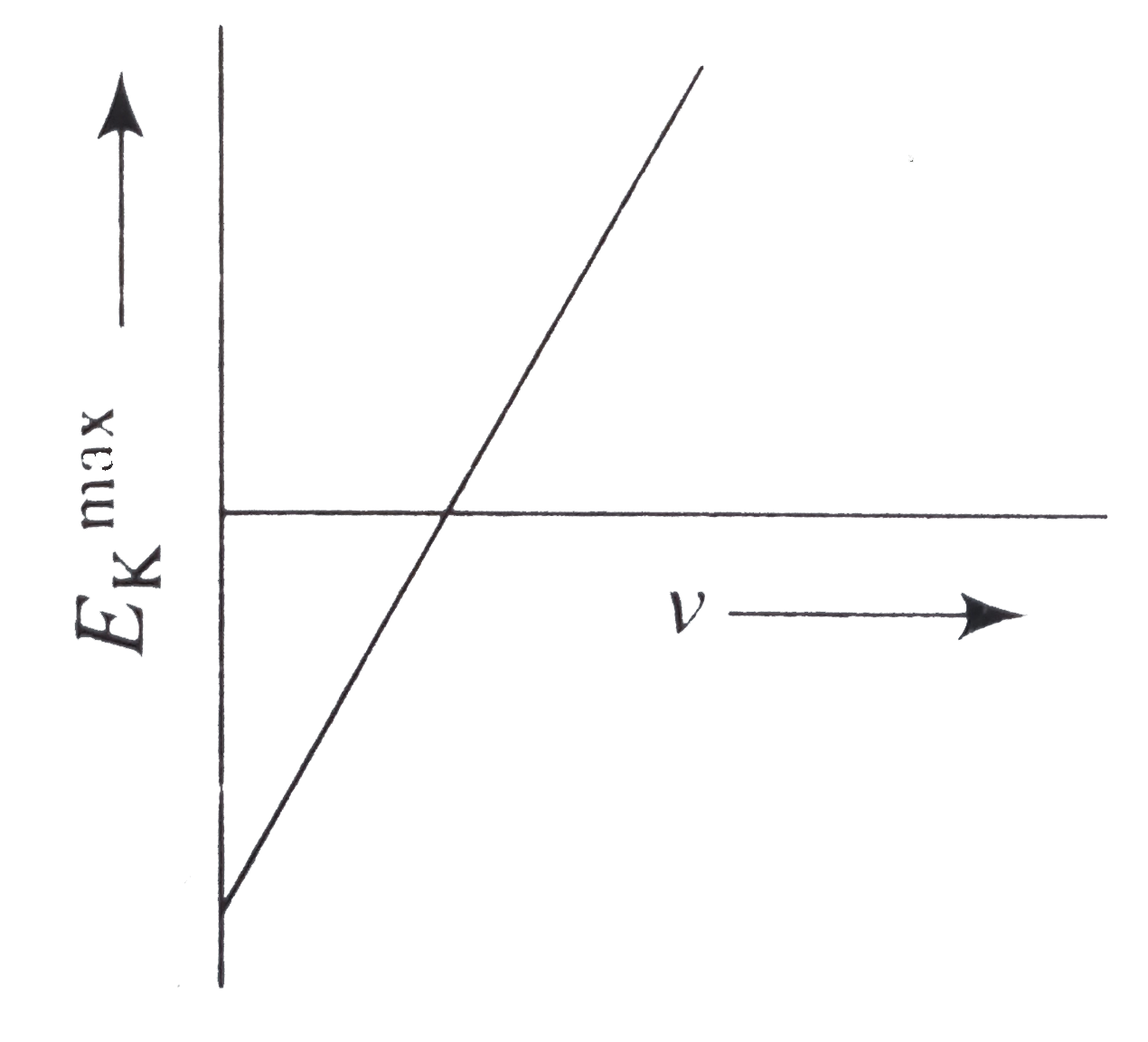
The maximum kinetic energy of the emitted photoelectrons against frequency υ of incident radiation is plotted as shown in Fig. This graph help us in determining the following physical quantities
charge on electron
work function of emitter
Planck's constant
ratio of Planck's constant and chargeon electron
The correct Answer is:C
Step by step video, text & image solution for The maximum kinetic energy of the emitted photoelectrons against frequency upsilon of incident radiation is plotted as shown in Fig. This graph help us in determining the following physical quantities by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- DUAL NATUREBook:NARAYNAChapter:DUAL NATUREExercise:LEVEL-I (C.W)
Explore 16 Videos
- DUAL NATUREBook:NARAYNAChapter:DUAL NATUREExercise:LEVEL-II (C.W)
Explore 20 Videos
- DUAL NATUREBook:NARAYNAChapter:DUAL NATUREExercise:LEVEL-II (H.W)
Explore 17 Videos
- DUAL NATURE OF RADIATION AND MATTERBook:NARAYNAChapter:DUAL NATURE OF RADIATION AND MATTERExercise:ASSERTION AND REASON
Explore 15 Videos
- ELECTRIC CHARGES AND FIELDSBook:NARAYNAChapter:ELECTRIC CHARGES AND FIELDSExercise:EXERCISE -4
Explore 43 Videos
Similar Questions
The frequency of a photon associated with an energy of 3.31eV is ( given h=6.62×10−34Js)
View SolutionIf light of wavelength λ1 is allowed to fall on a metal , then kinetic energy of photoelectrons emitted is E1. If wavelength of light changes to λ2 then kinetic energy of electrons changes to E2. Then work function of the metal is
View SolutionWhen a metal surface is illuminated by a monochromatic light of wave-length λ, then the potential difference required to stop the ejection of electrons is 3V. When the same surface is illuminated by the light of wavelength 2λ, then the potential difference required to stop the ejection of electrons is V. Then for photoelectric effect, the threshold wavelength for the metal surface will be
View SolutionAssertion : The energy (E) and momentum (p) of a photon are related by p=E/c.
Reason : The photon behaves like a particle.
View SolutionAn electron moving towards the east enters a magnetic field directed towards the north. The force on the electron will be directed
View SolutionThe kinetic energy of photoelectrons emitted by a photosensitive surface depends on the internsity of the incident radiation
View SolutionThe maximum kinetic energy of the emitted photoelectrons against frequency upsilon of incident radiation is plotted as shown in Fig. This graph help us in determining the following physical quantities
View SolutionFor the photoelectric effect, the maximum kinetic energy Ek of the emitted photoelectrons is plotted against the frequency ν of the incident photons as shown in the figure. The slope of the curve gives
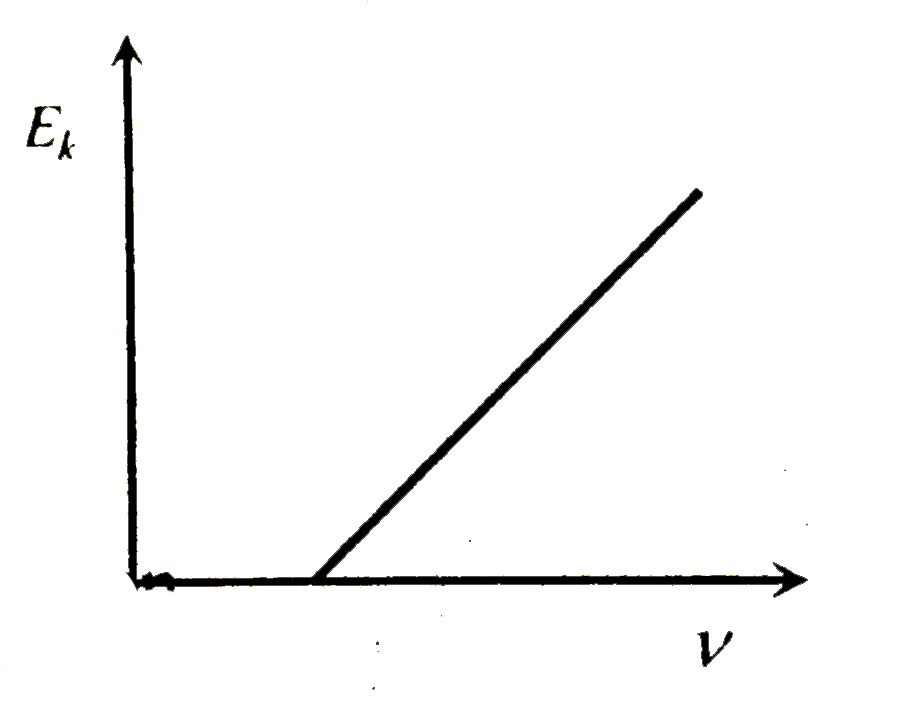
View SolutionA plot of kinetic energy of the emitted electron against frequency of the incident radiation yields a straight line.t he slope of the straight line is:
View SolutionThe kinetic energy (E_(k)) of a photoelectron varies with the frequency (v) of the incident radiation as which of the following graph ?
View SolutionThe kinetic energy E_(k) of a photoelectron varies with the frequency upsilon of the incident radiation as which of the following ?
View SolutionKinetic energy of surface photoelectrons is x when frequency of incident radiation is v .If frequency of incident radiation is made 2v, the kinetic energy of surface photoelectron will be
View Solution2.0times10^16Hz The kinetic energy of a photoelectron emitted from the surface of a particular metal by a radiation of frequency 1.25times10^16Hz Twice the kinetic energy of photoelectrons emitted by frequency radiation. Determine the starting frequency of the metal
View SolutionIf frequency of radiation incident on a photosensitive surface is doubled, the maximum kinetic energy of emitted photoelectrons also gets doubled.
View SolutionPlot a graph showing variation of stopping potential (V0) with the frequency (v) of the incident radiation for a given photosensitive material. Hence, state the significance of the threshold frequency in photoelectric emission.
Using the principle of energy conservation, write the equation relating the energy of incident photon, threshold frequency and the maximum kinetic energy of the emitted photoelectrons.
View Solutionउत्सर्जित फोटो - इलेक्ट्रॉनों की अधिकतम गतिज ऊर्जा और आपतित विकीर्ण की आवृत्ति के बीच ग्राफ बनाया जाता है इस ग्राफ की ढाल से कौन - सा भौतिक नियतांक निर्धारित किया जा सकता है ?
View Solution
