Tert-butyl chloride preferably undergo hydrolysis by
A
SN1 mechanism
B
SN2 mechanism
C
Any of (a) and (b)
D
None of these
Video Solution
Text Solution
The correct Answer is:A
Tertiary halide preferentially undergo SN1 substitution as they give stable carbocation.
H3C−CH3| C | CH3−Cltert- butyl chlorideSlow−−→−Cl(H3C)3C+Carbocation+OH−−−−−→fast(H3C)3COHt - butyl alcohol
|
Topper's Solved these Questions
- ALKYL HALIDEBook:MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERSChapter:ALKYL HALIDEExercise:PRACTICE EXERCISE (Exercise 2 (MISCELLANEOUS PROBLEMS))
Explore 70 Videos
- ALKYL HALIDEBook:MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERSChapter:ALKYL HALIDEExercise:MHT CET Corner
Explore 25 Videos
- ALKYL HALIDEBook:MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERSChapter:ALKYL HALIDEExercise:MHT CET Corner
Explore 25 Videos
- ALKANESBook:MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERSChapter:ALKANESExercise:EXERCISE 2
Explore 20 Videos
- BASIC PRINCIPLES AND TECHNIQUES IN ORGANIC CHEMISTRYBook:MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERSChapter:BASIC PRINCIPLES AND TECHNIQUES IN ORGANIC CHEMISTRYExercise:EXERCISE 2
Explore 20 Videos
Similar Questions
Knowledge Check
Tertiary butyl chloride preferably undergo hydrolysis by:
ASN1 mechanismBSN2 mechanismCand of (a) and (b)Dnone of theseTert - butyl chloride is
ACH3−CH2−CllCH−CH3BCH3−CH2−CH2−CH2−ClCCH3−CH∣CH3−CH2−ClDCH3−CH3∣C∣CH3−ClIsopropyl chloride undergoes hydrolysis by :
ASN1 mechanismBSN2 mechanismCSN1 and SN2 mechanismDNeither SN1 nor SN2 mechanism
Iso-propyl chloride undergoes hydrolysis by
View SolutionSecondary butyl chloride will undergo alkaline hydrolysis in the polar solvent by the mechnism
View SolutionIsopropyl chloride undergoes hydrolysis by :
View Solution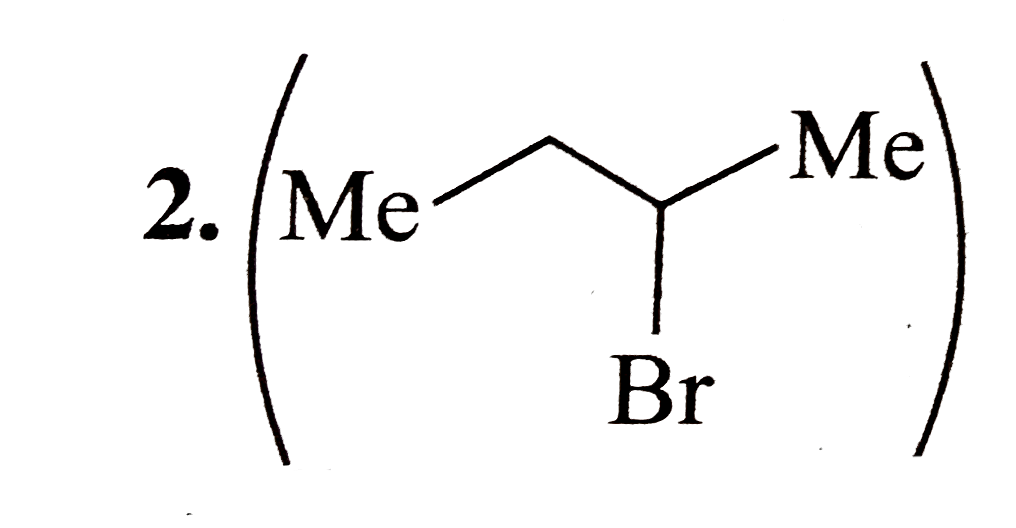 (sec-Butyl bromide) undergoes alkaline hydrolysis by :
(sec-Butyl bromide) undergoes alkaline hydrolysis by :
View SolutionAssertion : Mercuric chloride becomes red on adding KI solution.
Reason : Mercuric chloride undergoes hydrolysis forming basic chloride.
View Solution
