Consider the following reaction,
H2C=CH2+Br2BA−−→BrCH2CH2BrC
The reactant 'A' and colour of 'B' and 'C' are respectively
A
A→CCl4B→colourless C→reddish brown
B
A→CCl4B→reddish brownC rarr colourless
C
A→CBr4 B → colourless C → reddish brown
D
A→CBr4 B → reddish brown C → colourless
Video Solution
Text Solution
The correct Answer is:B
Addition of bromine in CCl4 to an alkene resulting in discharnge of reddish brown colour of bromine constitutes an important method for detection of double bond in a molecule. The addition results in the synthesis of vic-dibromides, which is colourless.

|
Similar Questions
Knowledge Check
CH3−C≡C−CH3(1)H2/Pt−−−−−−→(2)Br2X
A(d)-2, 3-DibromobutaneB(l)-2, 3-DibromobutaneC(dl)-2, 3-DibromobutaneDmeso-2, 3-DibromobutaneIn the following reaction order , B is
CH3CH2COOHP/Br2−−−→AalcKOH−−−−→H+BACH2=CH−COOHBCH2−CH∣Br−COOHCCH3CH2COBrDCH3CH2CH2OHConsider the following reaction
H2C=CH−CH2CH2−OHBr2/CCl4−−−−−→ADil.KOH−−−−−→25∘CB
The product B is:A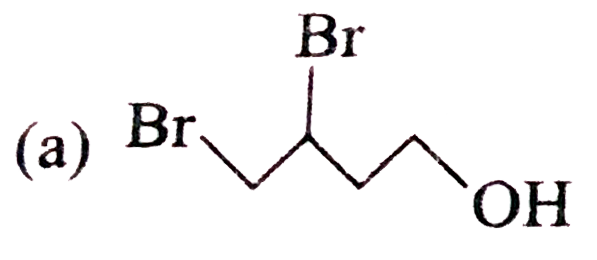 B
B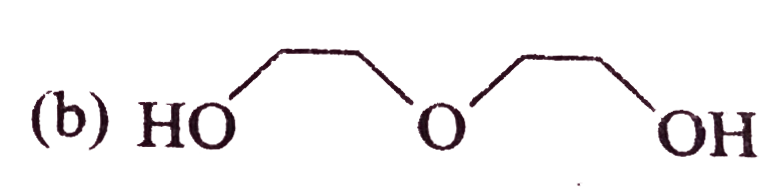 C
C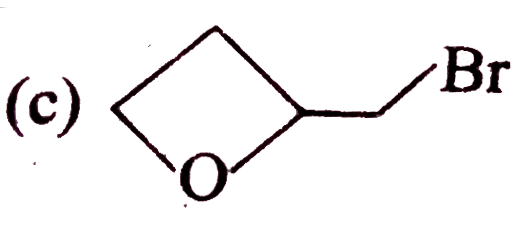 D
D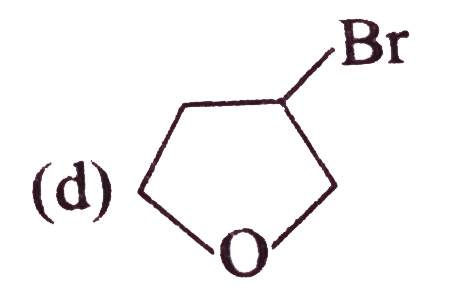
CH3−C≡C−CH3H2−−−−−−→Pd−BaSO4
(A) Br2−−→CCl4(B)
View Solution(CH3)2C=CH2Br2−−→H2O
The major product of the reaction is
View SolutionIn the reaction:
H2C=CH−CH=CH2Br2−−−→180∘C[X], [X] will be
View SolutionConsider the following sequence of reactions
C2H5C≡CHCH3MgBr−−−−−→A(1)HCHO−−−−−−→(2)H2O+B
The product (B) is
View SolutionH3C−CH2−C∣BrH−CH3NaNH2−−−−→
Which are correct statement?
View Solution
