On heating an organic compound with dry CuO, a gas is liberated which turns the line water milky . The gas is ______.
H2
O2
CO2
N2
The correct Answer is:C
Step by step video, text & image solution for On heating an organic compound with dry CuO, a gas is liberated which turns the line water milky . The gas is ______. by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- BASIC PRINCIPLES AND TEACHNIQUES IN ORGANIC CHEMISTRYBOOK - TARGET PUBLICATIONCHAPTER - BASIC PRINCIPLES AND TEACHNIQUES IN ORGANIC CHEMISTRYEXERCISE - Critical Thinking
46 Videos
- BASIC PRINCIPLES AND TEACHNIQUES IN ORGANIC CHEMISTRYBOOK - TARGET PUBLICATIONCHAPTER - BASIC PRINCIPLES AND TEACHNIQUES IN ORGANIC CHEMISTRYEXERCISE - Competitive Thinking
53 Videos
- ALKANESBOOK - TARGET PUBLICATIONCHAPTER - ALKANESEXERCISE - ANSWERS TO EVALUATION TEST
5 Videos
- HYDROGEN BOOK - TARGET PUBLICATIONCHAPTER - HYDROGEN EXERCISE - Evaluation test
14 Videos
Similar Questions
Knowledge Check
Mark the gas which turns lime water milky
AH2SBSO2CCl2DCO2Which of them liberate a gas which turns lime water milky .
A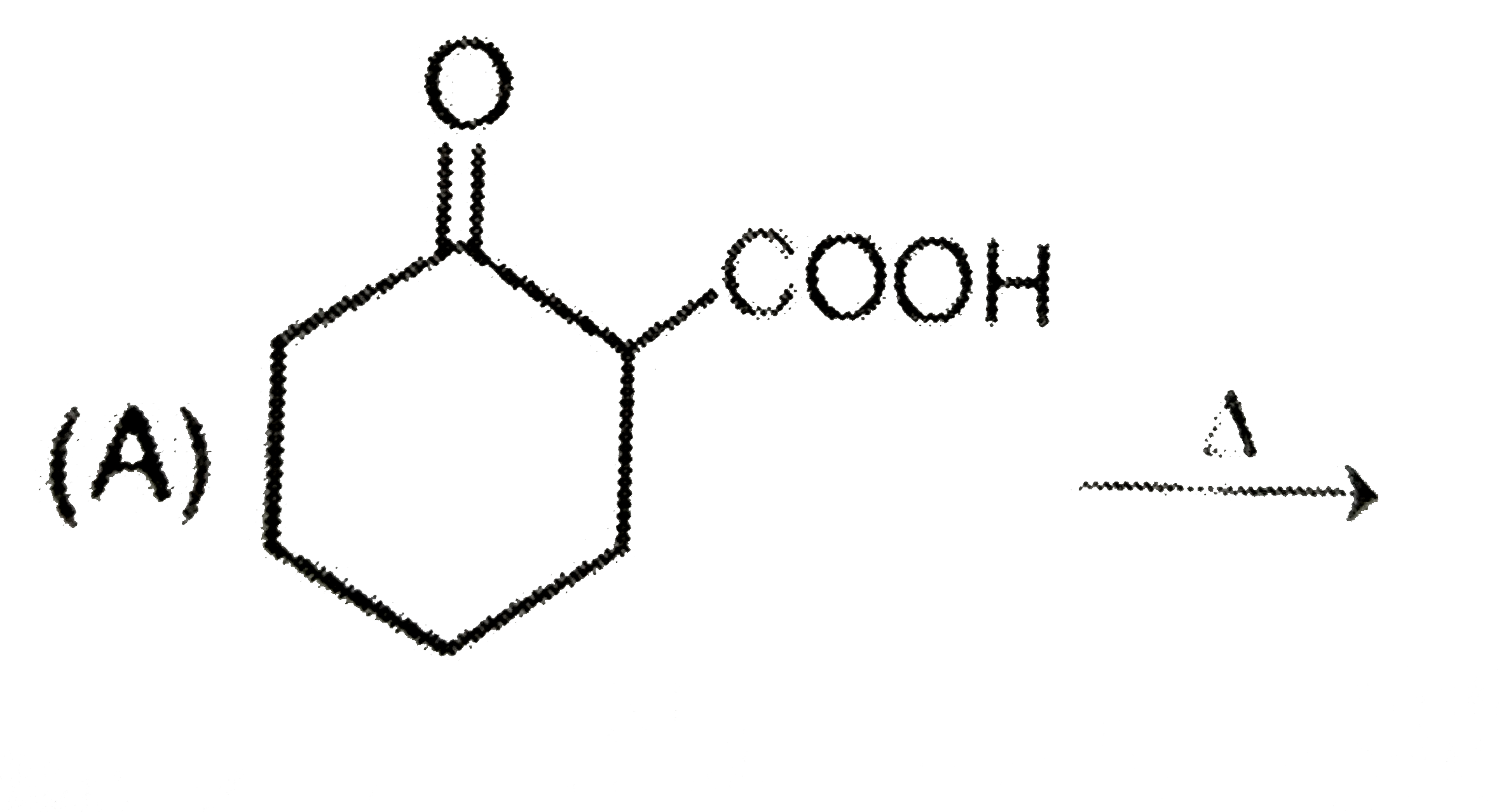 BCH3−CH−COOH | COOHΔ−−→CHOOC−(CH2)5−COOHCa(OH)2/Δ−−−−−−−−→D
BCH3−CH−COOH | COOHΔ−−→CHOOC−(CH2)5−COOHCa(OH)2/Δ−−−−−−−−→D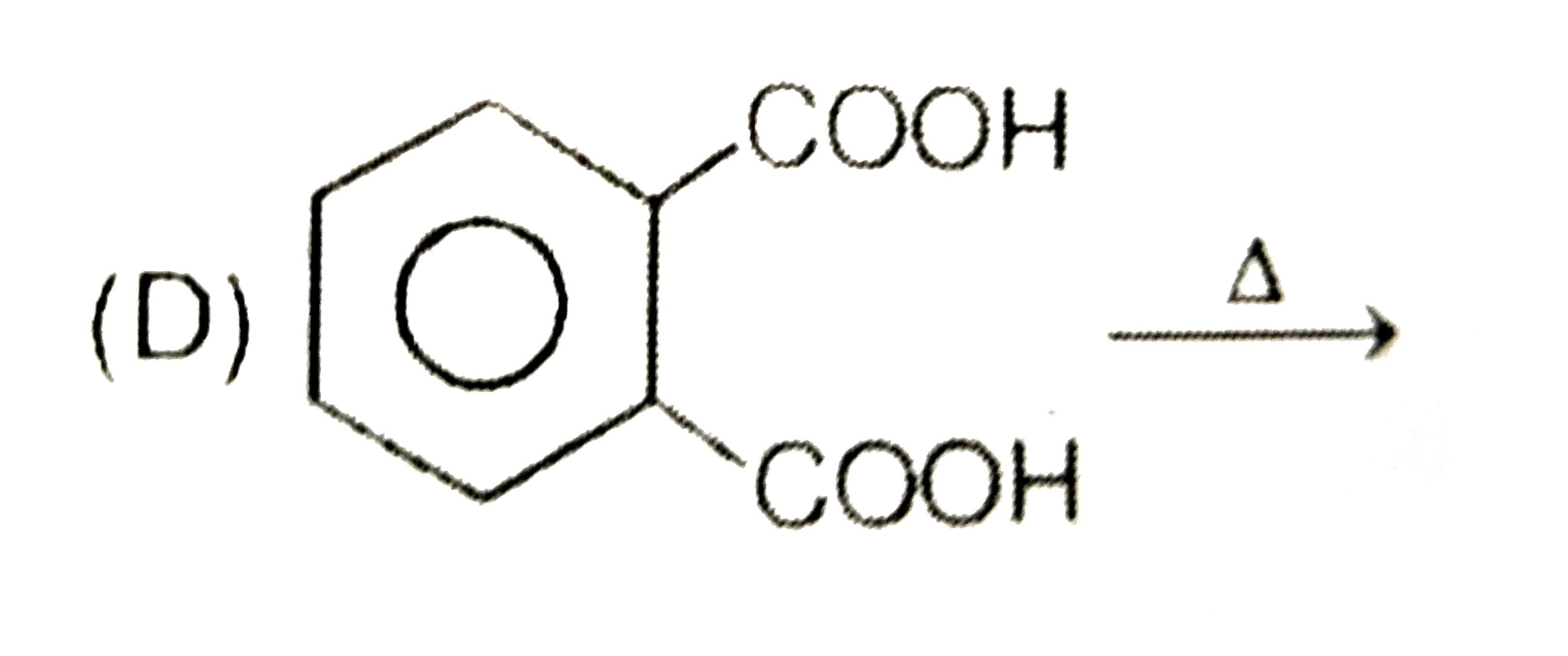
The gas that turns lime water milky is
ACO2BSO2CBoth of theseDNone of these
If (X) turn line water milky , then X may be
View SolutionWhich of the following gases turn lime water milky?
View SolutionOn treated with dilute H2SO4, a salt gives a gas whch turns lime water milky. The salt can be
View SolutionIn qualitive analysis , an organic compound'A' is heated Dry CuO,CO2 gas is liberated and H2O is formed . The same compound 'A' when heated with Na2O2 , followed by boiling with HNO3 and ammonium molybdate gives yellow precipitate . Based on teh tests , the elements present in compound 'A' are __________.
View SolutionA solution reacts with marble chips to produce a gas which turns lime water milky. The solution contains:
View Solution

