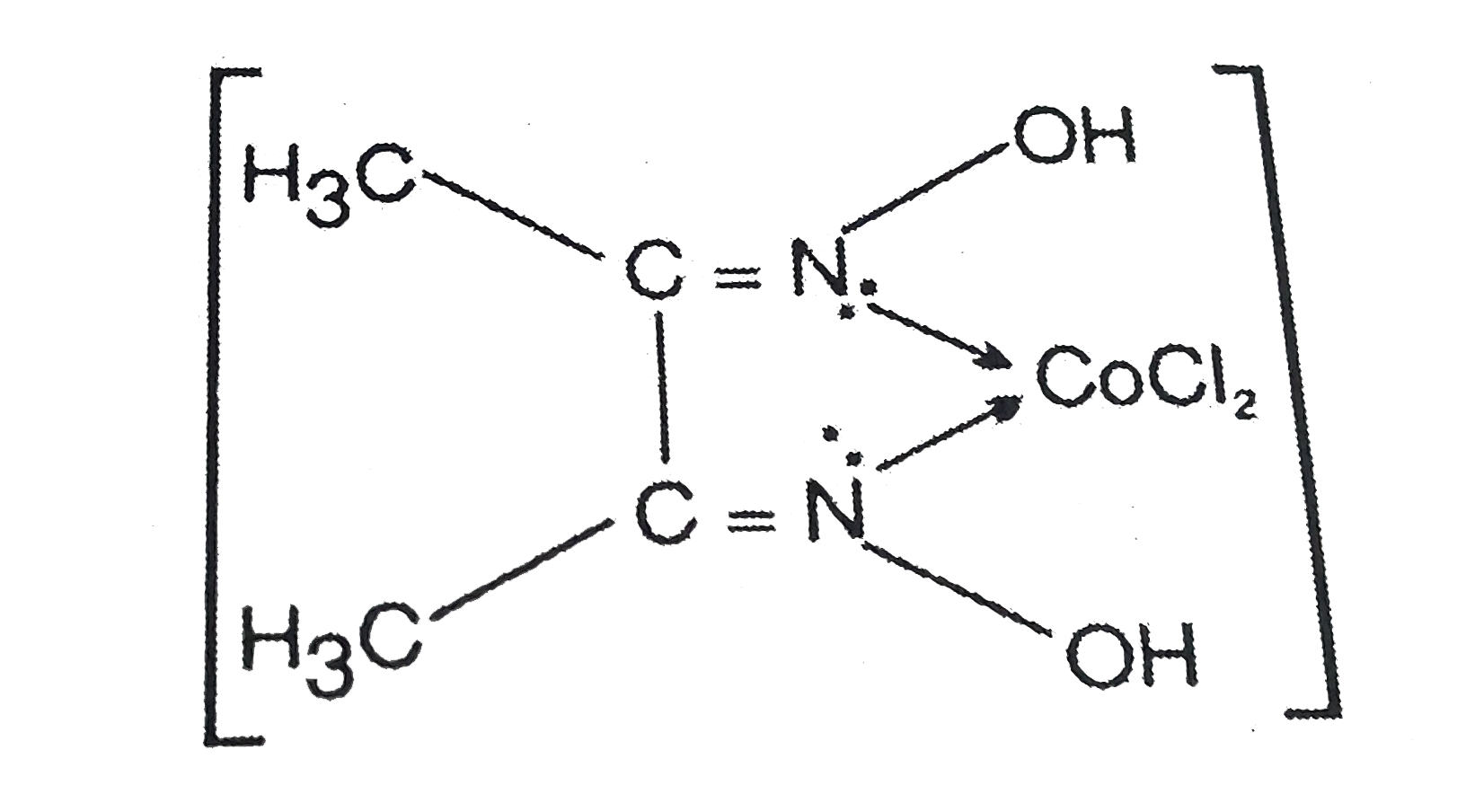
The IUPAC name of the complex ion formed when gold dissolves in aqua-regia is
A
tetrachloridoaurate (III)
B
tetrachloridoaurate (I)
C
tetrachloridaurate (II)
D
dichloridoaruate (III)
Video Solution
Text Solution
The correct Answer is:A
|
Answer
Step by step video, text & image solution for The IUPAC name of the complex ion formed when gold dissolves in aqua-regia is by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- COORDINATION COMPOUNDSBook:TARGET PUBLICATIONChapter:COORDINATION COMPOUNDSExercise:EVALUATION TEST
Explore 19 Videos
- COORDINATION COMPOUNDSBook:TARGET PUBLICATIONChapter:COORDINATION COMPOUNDSExercise:CRITICAL THINKING
Explore 51 Videos
- COMPOUND CONTAINING NITROGEN Book:TARGET PUBLICATIONChapter:COMPOUND CONTAINING NITROGEN Exercise:EVALUATION TEST
Explore 10 Videos
- D AND F-BLOCK ELEMENT Book:TARGET PUBLICATIONChapter:D AND F-BLOCK ELEMENT Exercise:EVALUATION TEST
Explore 15 Videos
Similar Questions
Knowledge Check
The IUPAC name of the complex ion formed when gold dissolves in aque-regia is
Atetrachloridoaurate (III)Btetrachloridoaurate (I)Ctetrachloridoaurate (II)Ddicoloridoaurate (III).The IUPAC name of the complex ion formed when gold dissolves in aqua-regia is
ATera chloridoaurte (II)BTetra chloridoaurate (I)Cdena chloridoaurate (II)DDichloridoaurate (III)Gold dissolves in aqua regia forming
AAu(NO3)2BH[AuCl4]CAuClDAuNO3

 .
.