The decreasing order of C-X bond length in CH3−X is
A
CH2I>CH3Br>CH3Cl>CH3F
B
CH3F>CH3Cl>CH3Br>CH3I
C
CH3F>CH3Cl>CH3I>CH3Br
D
CH3I>CH3Cl>CH3F>CH3Br
Video Solution
Text Solution
The correct Answer is:A
Nature of C-X bond
|
Similar Questions
Knowledge Check
The decreasing order of bond angle is
ANO2>NO+2>NO−2BNO−2>NO2>NO+2CNO+2>NO2>NO−2DNO+2>NO−2>NO2The correct order of C-O bond length among
ACO<CO2<CO2−3BCO2<CO2−3<COCCO<CO2−3<CO2DCO2−3<CO2<COThe decreasing order of O - O bonds length in the following molecules is:
ANa2O2>KO2>O2>O2(AsF6)BKO2=Na2O2>O2>O2(AsF6)CKO2>Na2O2>O2>O2(AsF6)DNa2O2>O2>KO2>O2(AsF6)
Correct decreasing order O-O bond length in O2,
View SolutionThe decreasing order of bond length of C=C bond in the following compounds is :
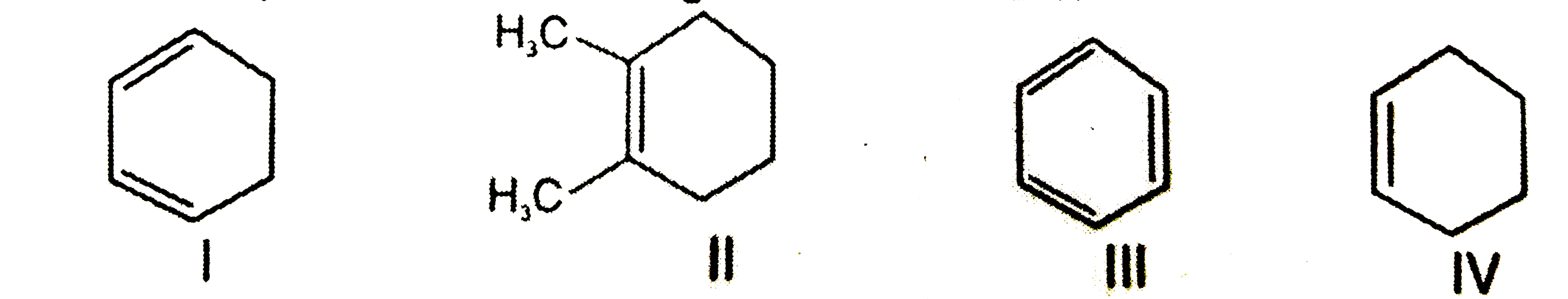
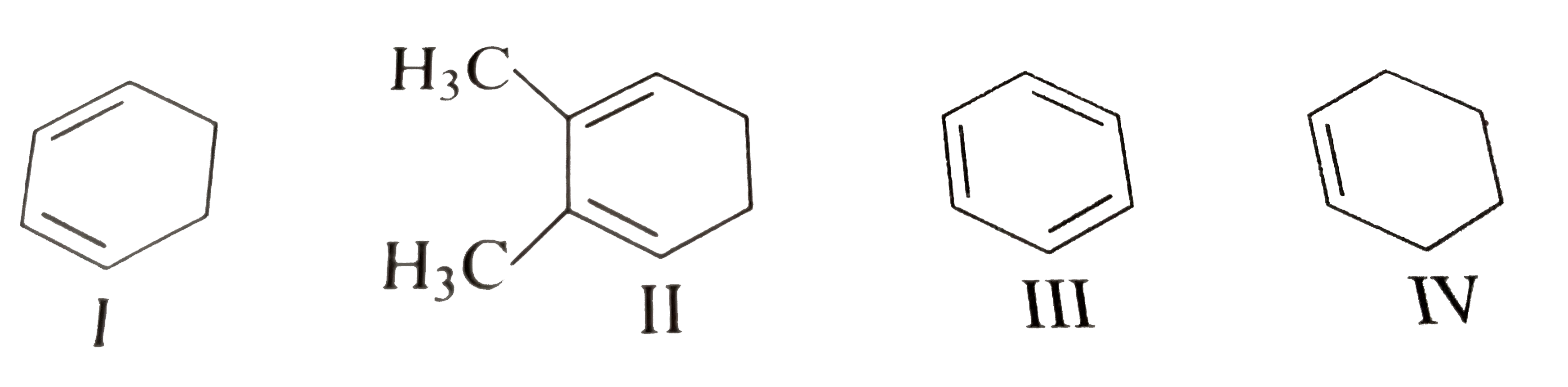
View SolutionThe decreasing order of bond length of C=C in the following compounds is :
View SolutionThe correct order of C–X bond polarity is:
View SolutionArrange the following in decreasing order of their C=C bond length
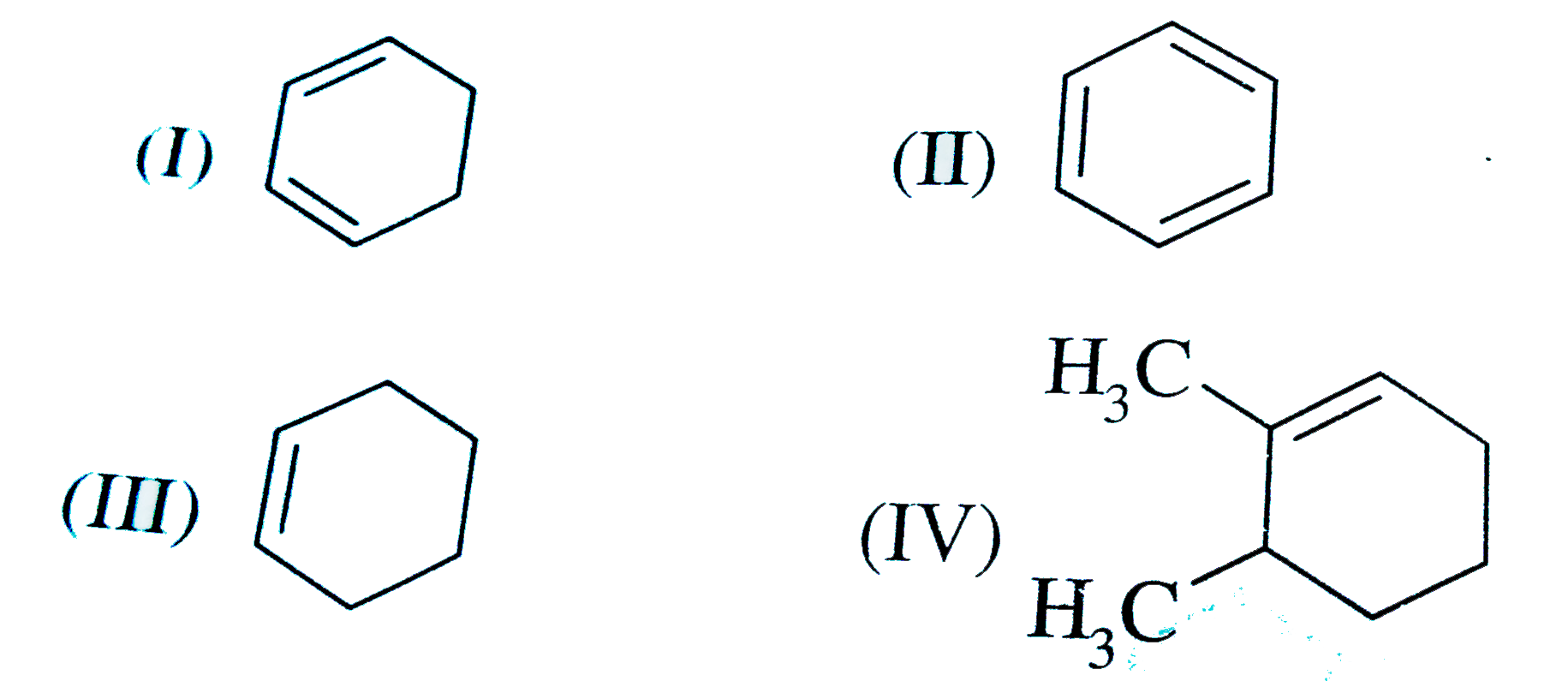
View Solution

