The most unlikely representation of resonance structures of p- nitrophenoxide ion is
A

B

C

D

Text Solution
The correct Answer is:C
In case of nitrogen atom the valence shell cannot have more than 8 electrons because of absence of d- orbitals in it.
|
Topper's Solved these Questions
- ORGANIC COMPOUNDS CONTAINGING NITROGEN BOOK - MARVEL PUBLICATIONCHAPTER - ORGANIC COMPOUNDS CONTAINGING NITROGEN EXERCISE - TEST YOUR GRASP
40 Videos
- HALOGEN DERIVATIVES OF ALKANES (AND ARENES)BOOK - MARVEL PUBLICATIONCHAPTER - HALOGEN DERIVATIVES OF ALKANES (AND ARENES)EXERCISE - TEST YOUR GRASP
15 Videos
- P-BLOCK ELEMENTS BOOK - MARVEL PUBLICATIONCHAPTER - P-BLOCK ELEMENTS EXERCISE - TEST YOUR GRASP
50 Videos
Similar Questions
Knowledge Check
The most unlikely representation of resonance structure of p-nitrophenoxide ion is :-
A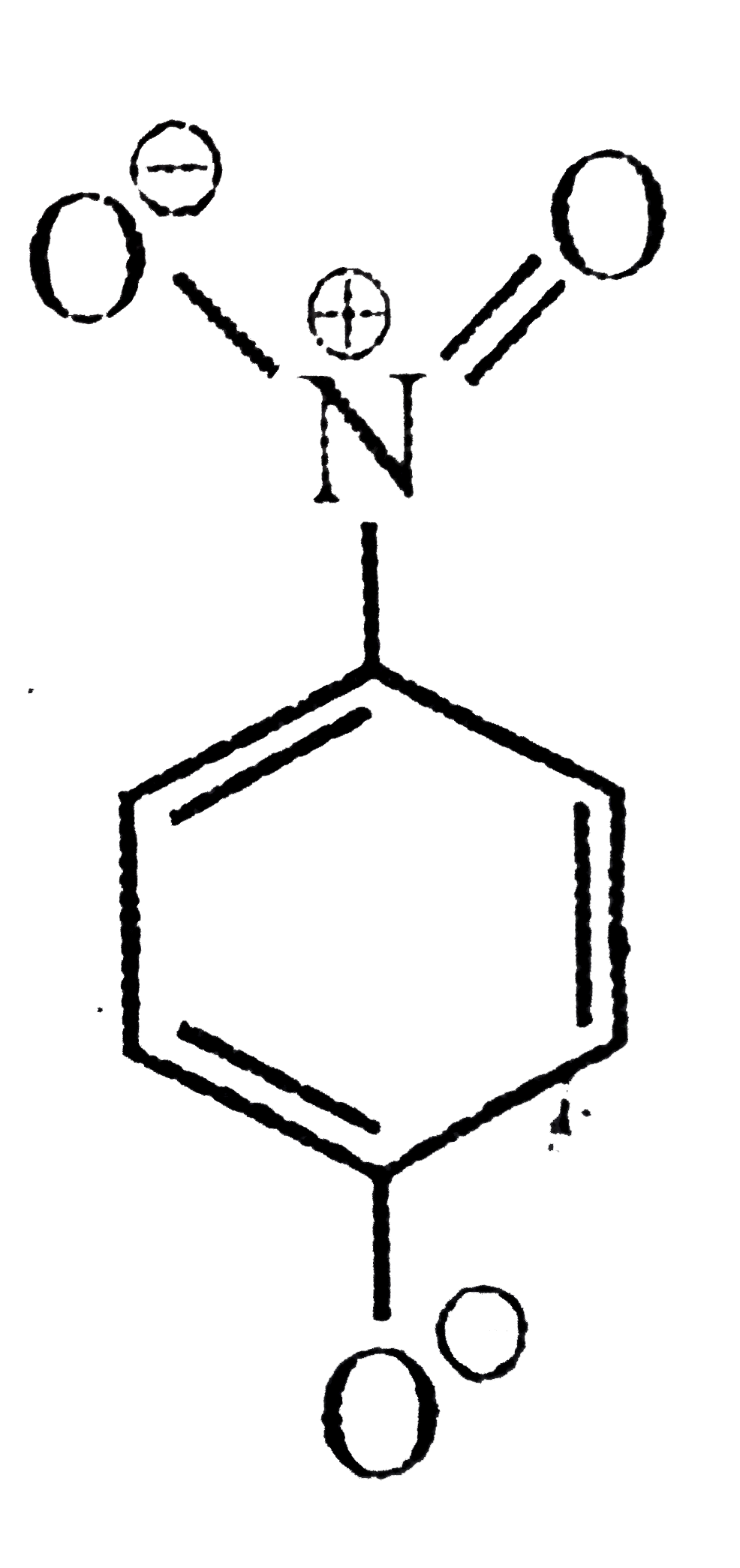 B
B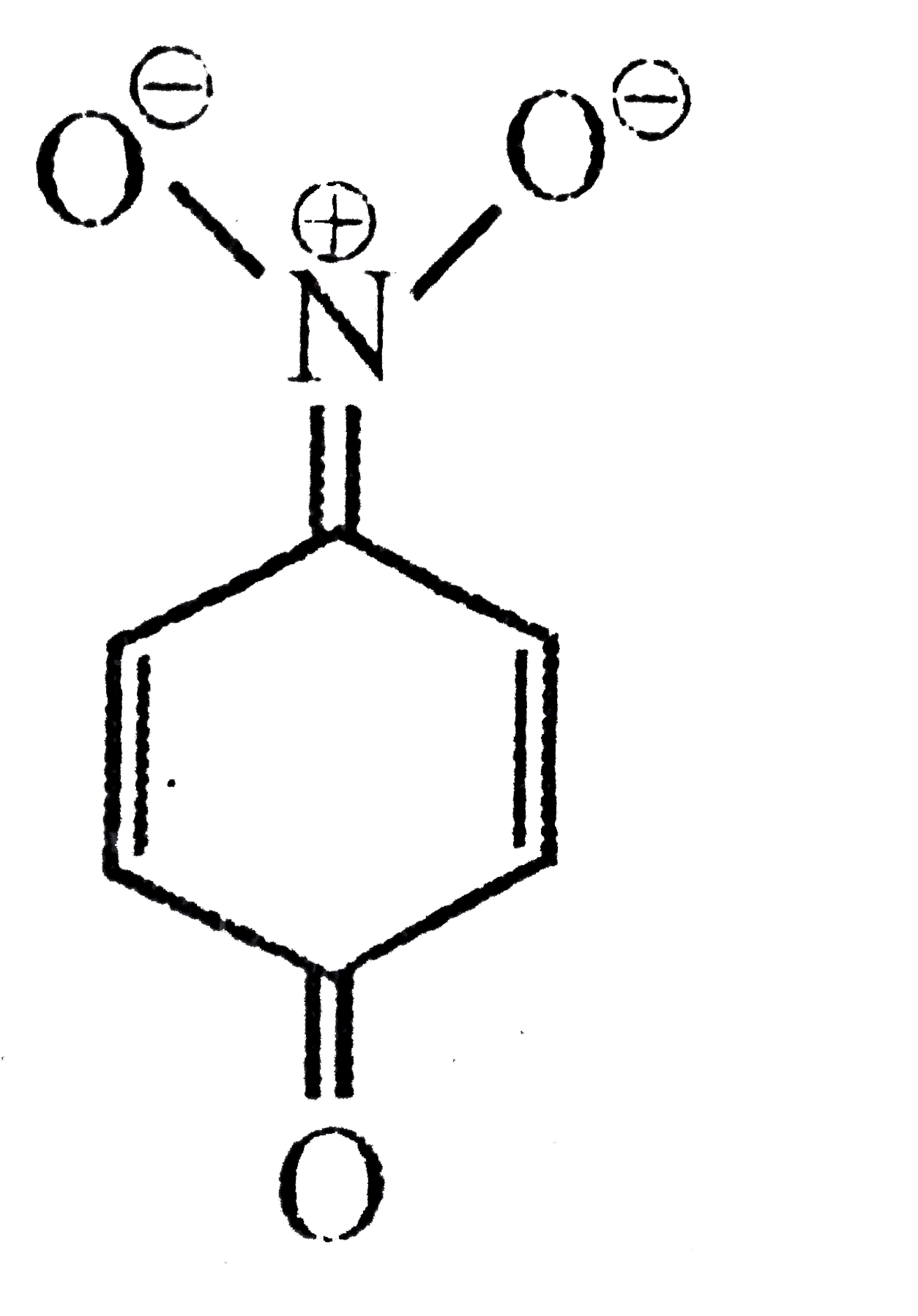 C
C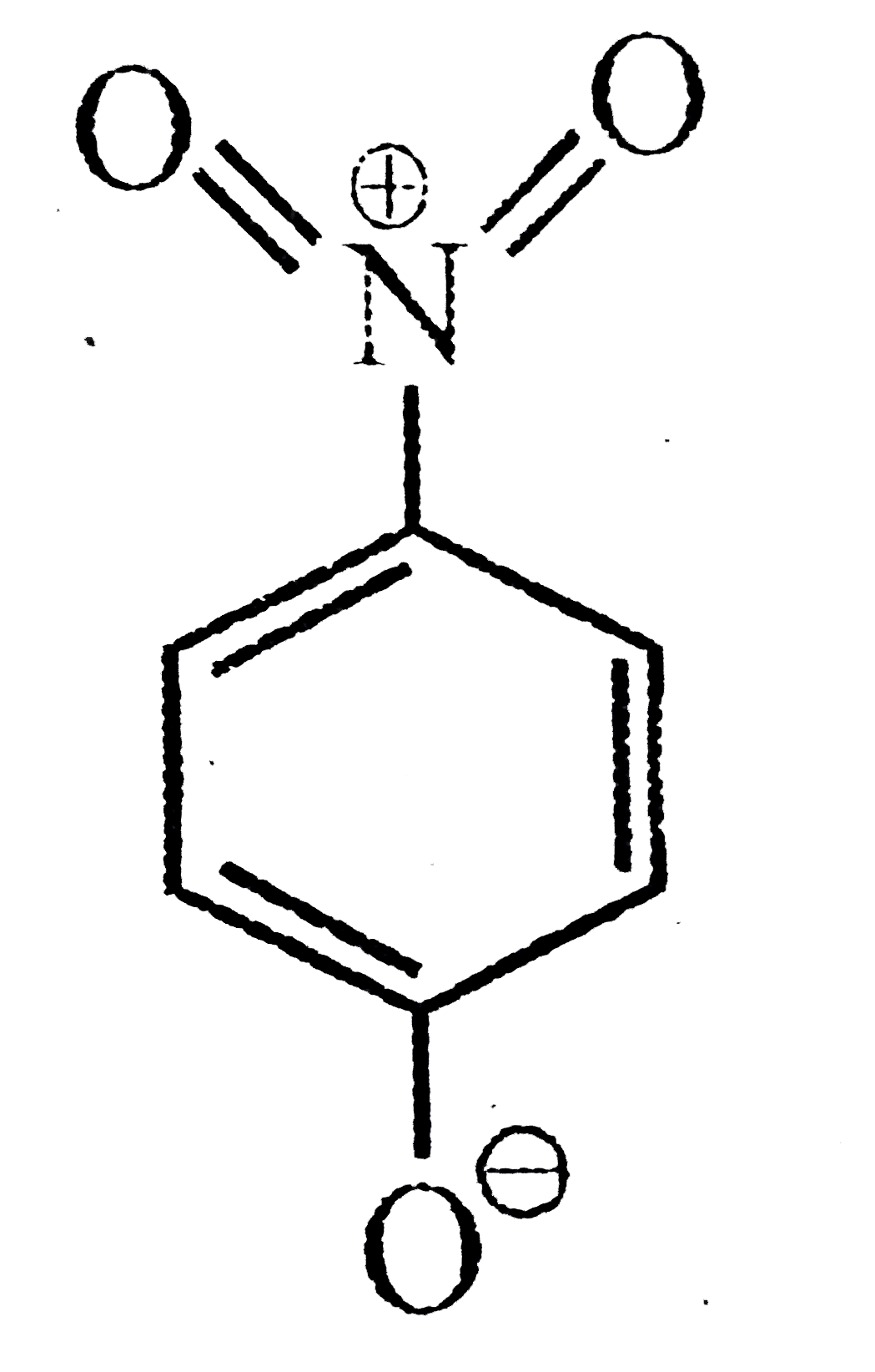 D
D
The most unlikely representation of resonance structure of p-nitrophenoxide ion is:
A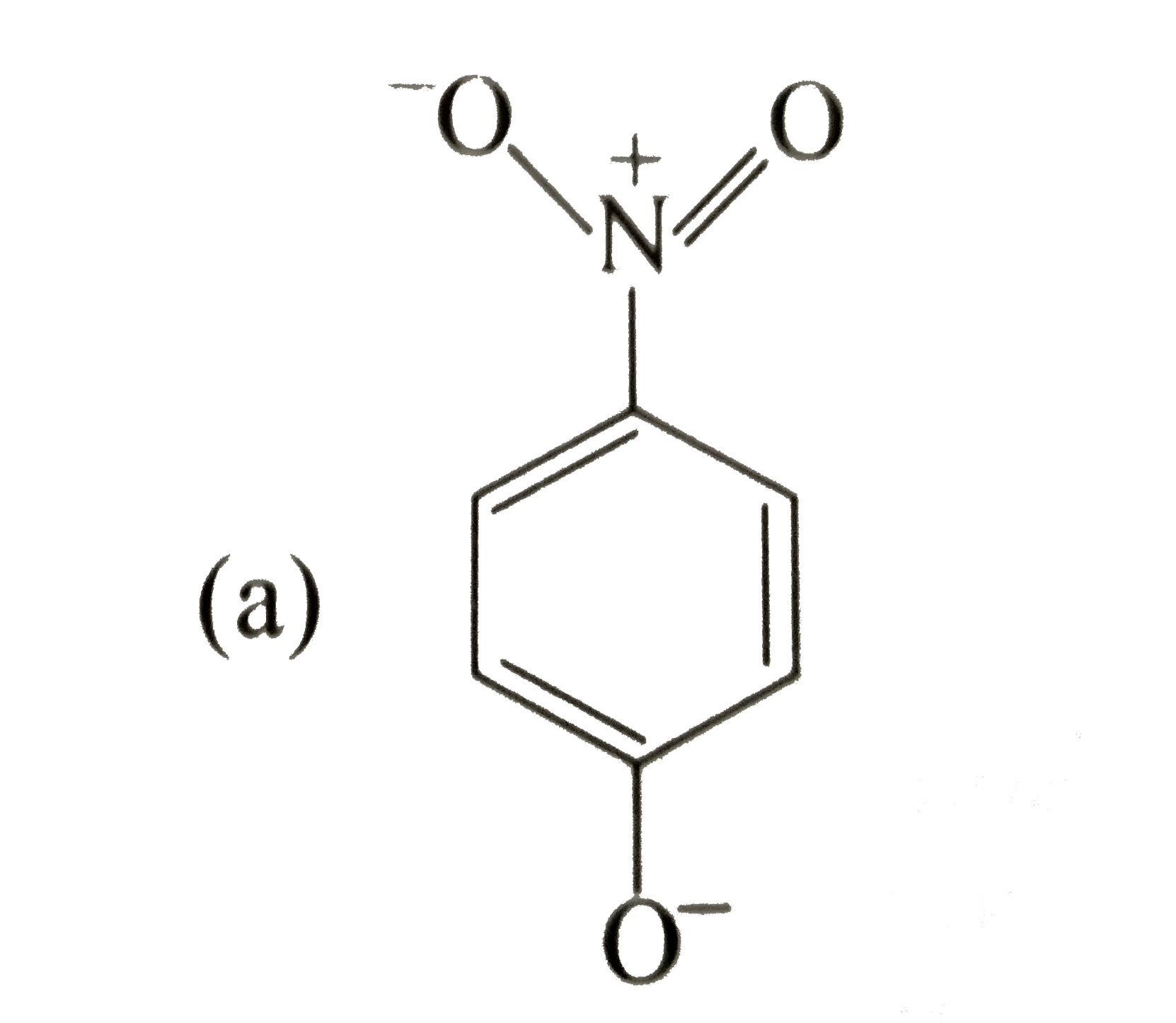 B
B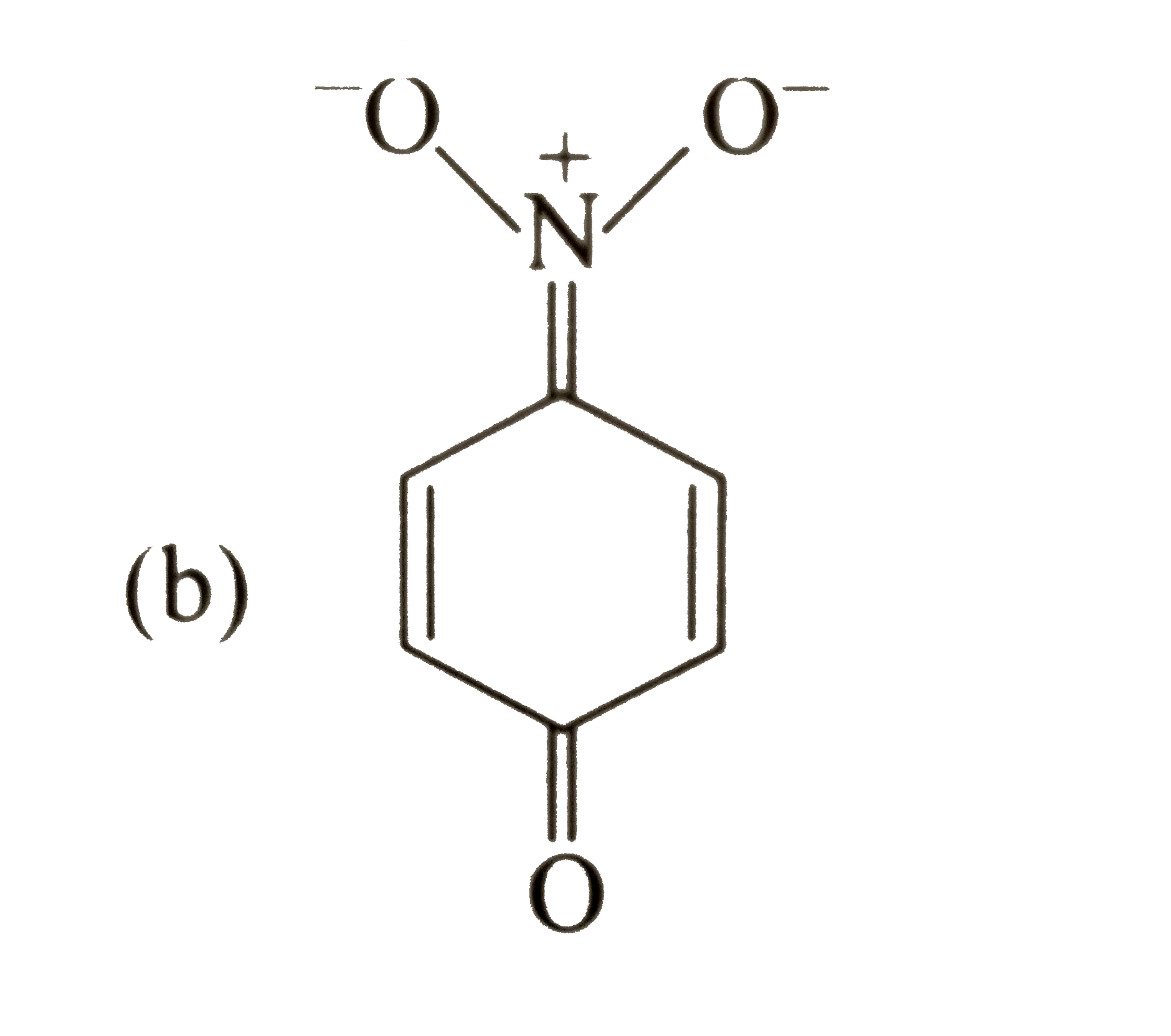 C
C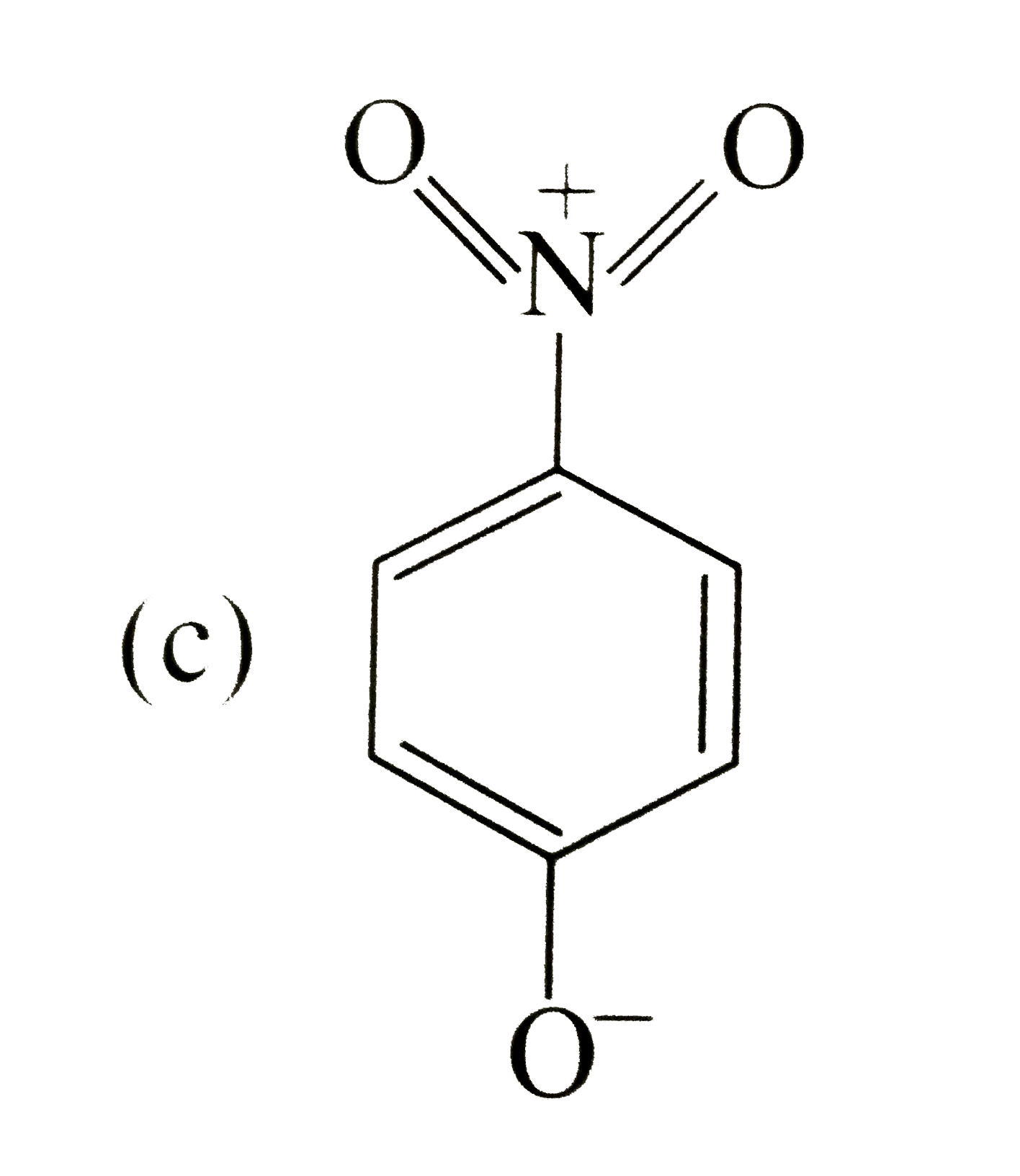 D
D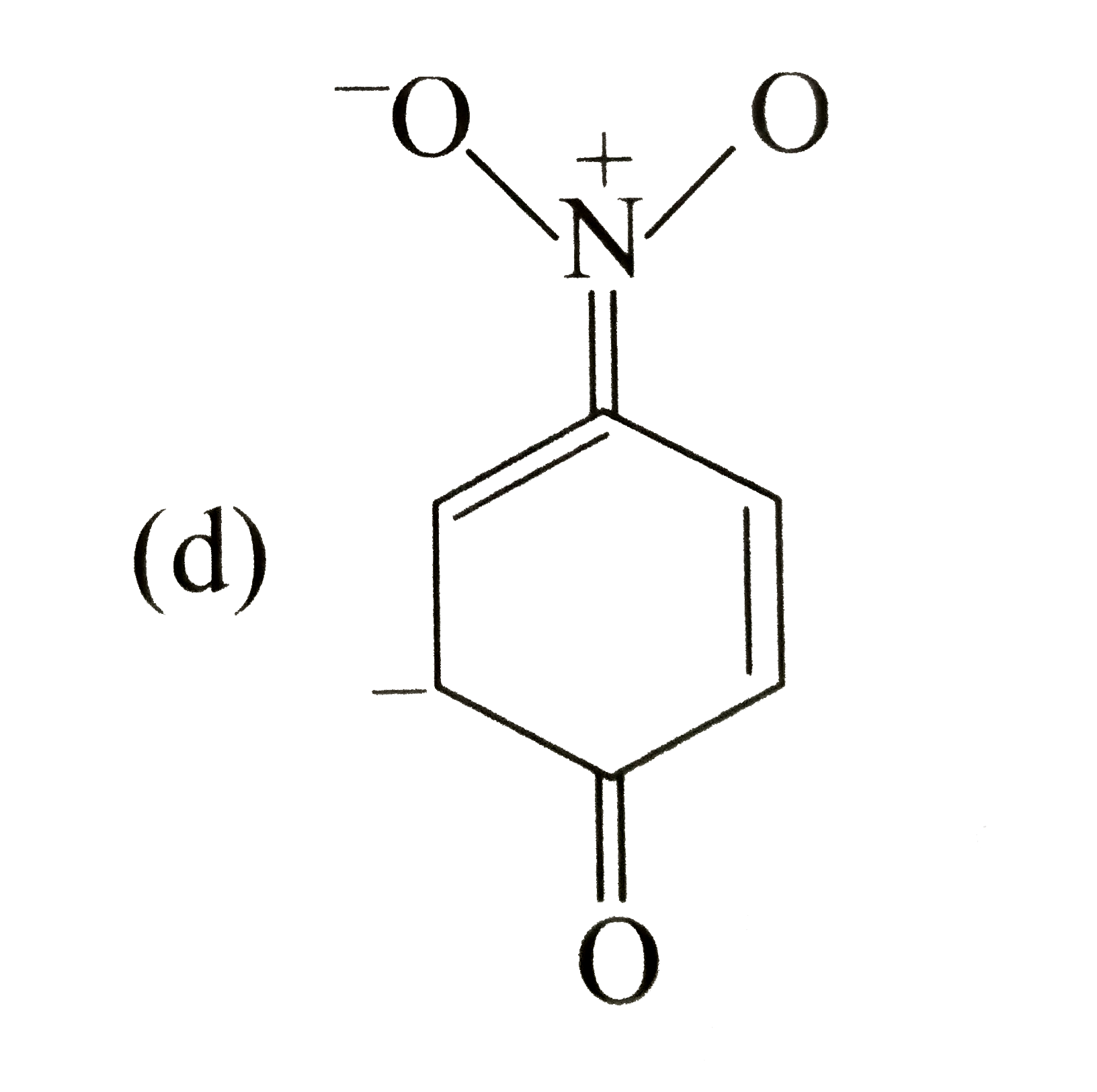
The most unlikely representation of resonance structure of p-nitrophenoxide ion is:
A B
B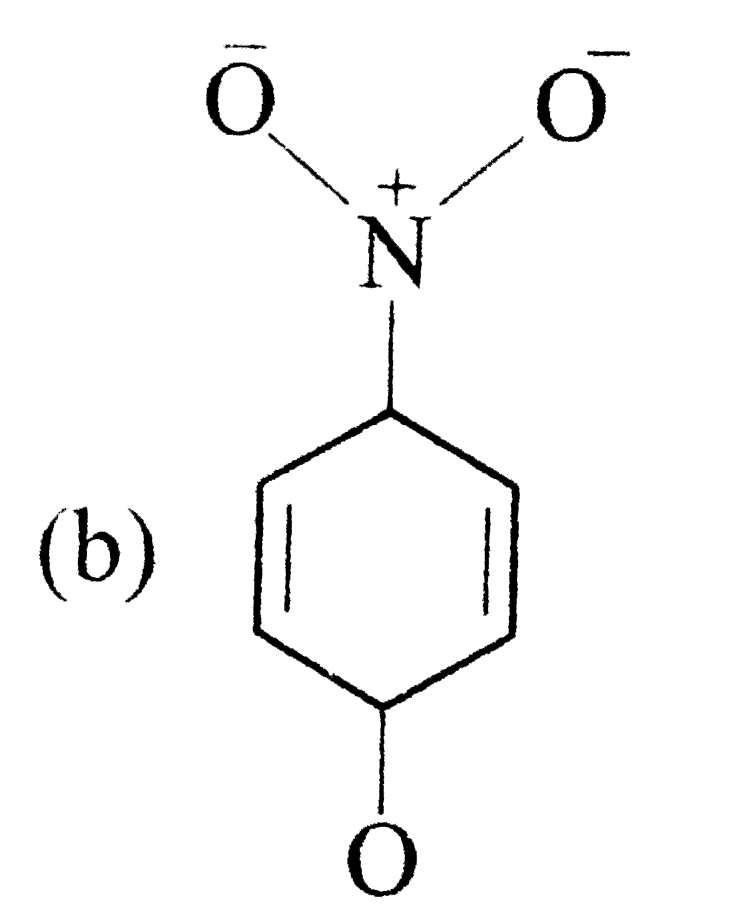 C
C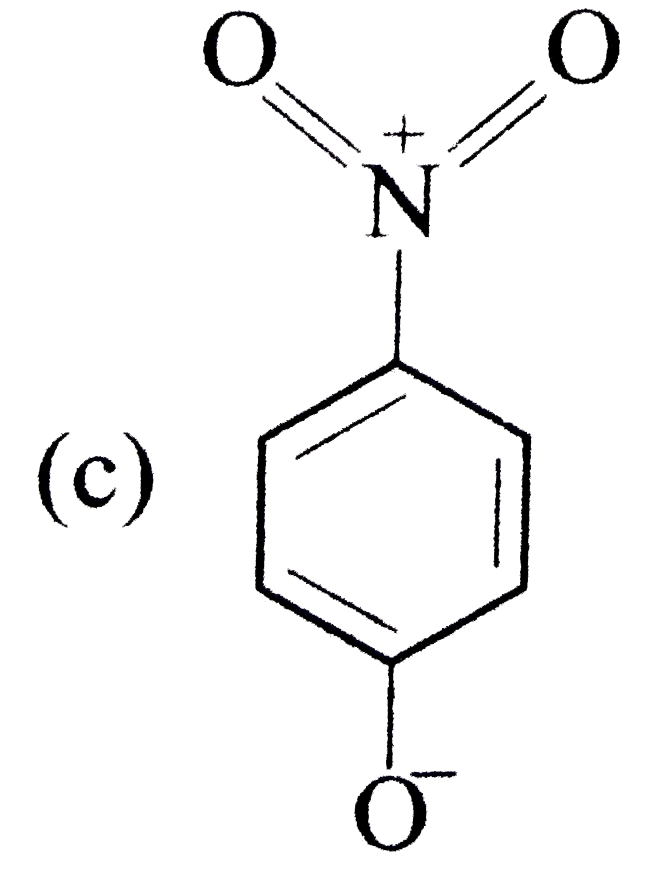 D
D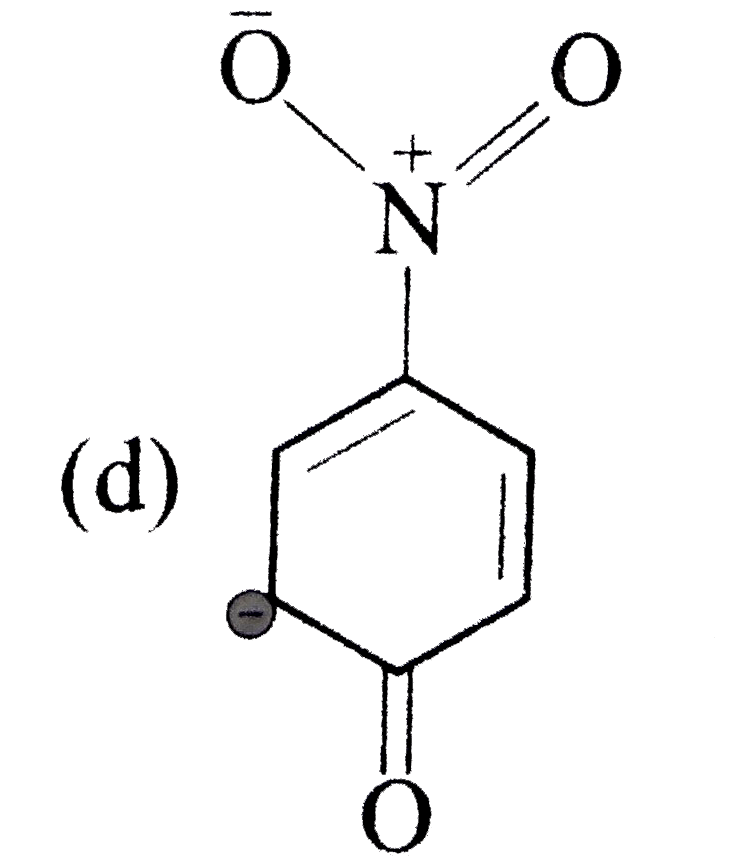
The most unlikely representation of resonance structure of p-nitrophenoxide is :
View SolutionThe most unlikely representation of resonance structure of p-nitrophenoxide is :
View SolutionThe most unlikely representation of resonance structuresof nitrophenoxide ion is
View SolutionThe most likely representation of resonance structure of p-nitrophenoxide is :
View SolutionThe most unlikely resonating structures of pnitrophenoxide ion is :
View Solution

