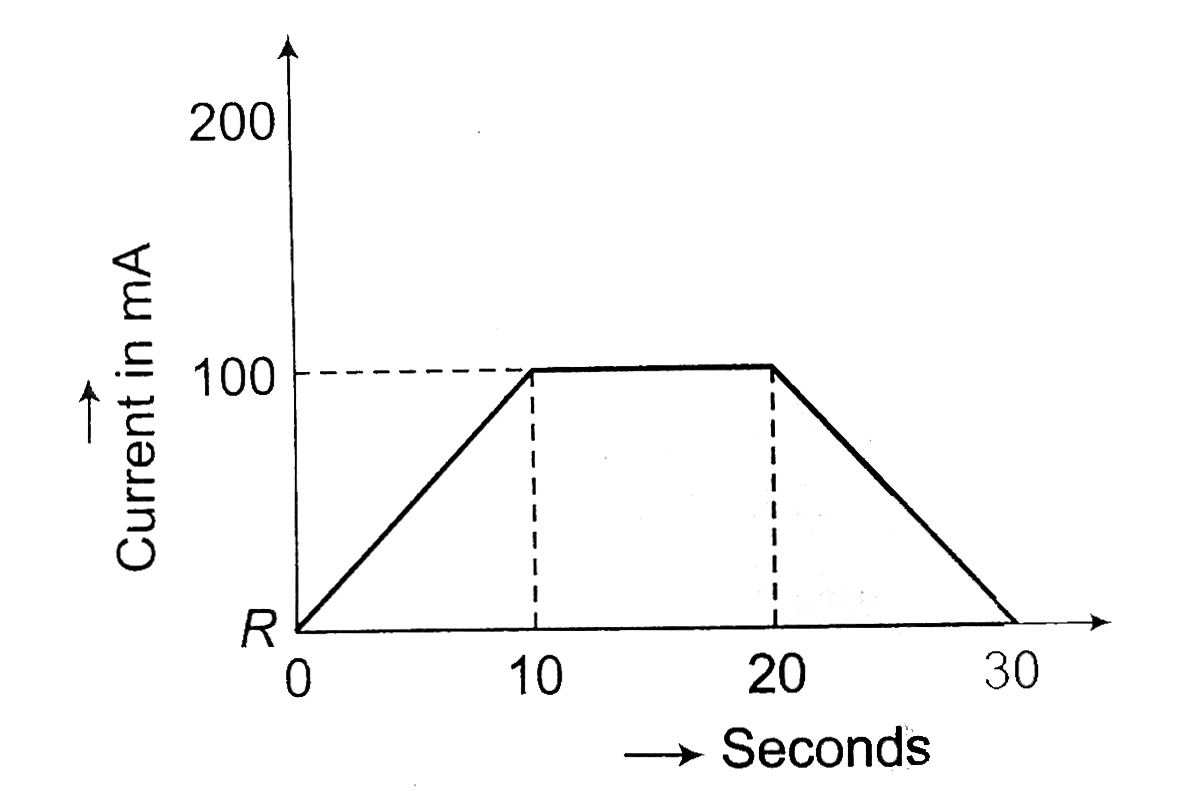
In a copper voltmeter, mass deposite in 30 seconds is 'm' gram. If the time-current graph is as shown in figure. ECE of copper is
m
m/2
0.1m
0.6m
The correct Answer is:B
Area of i-t graph gives charge
q=it=12(10+30)×100×10−3=2C
m=Zit=Z×2
Z=m/2.
Similar Questions
In a Cu - voltmeter, mass deposited in 30 s is m gm. If the time - current graph is shown in the following figure
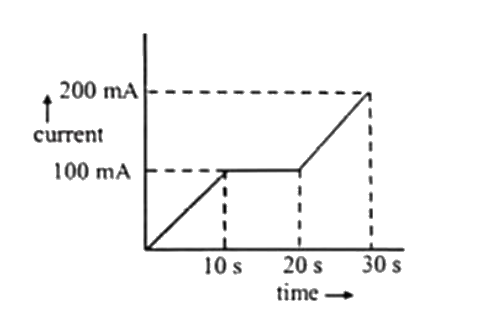
What is the electrochemical equivalent of Cu?
View SolutionIn a Cu-voltameter, mass deposited in 30s is 'm' g. If the time -current graph is shown in the following figure :
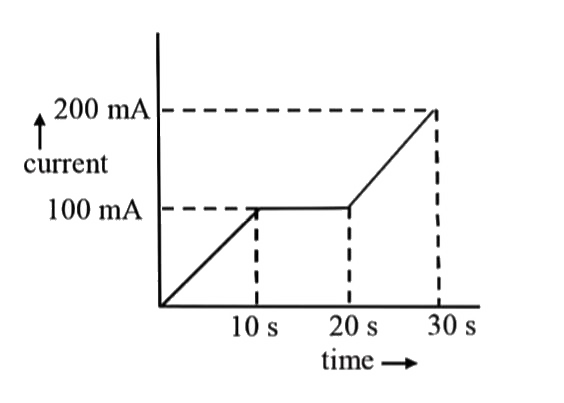
What is the electrochemical equivalent of Cu ?
View Solution
Knowledge Check
In a Cu-voltameter, mass deposited in 30s is m gm. If the time-current graph is shown in the following figure. What is the electrochemical equivalent of Cu?
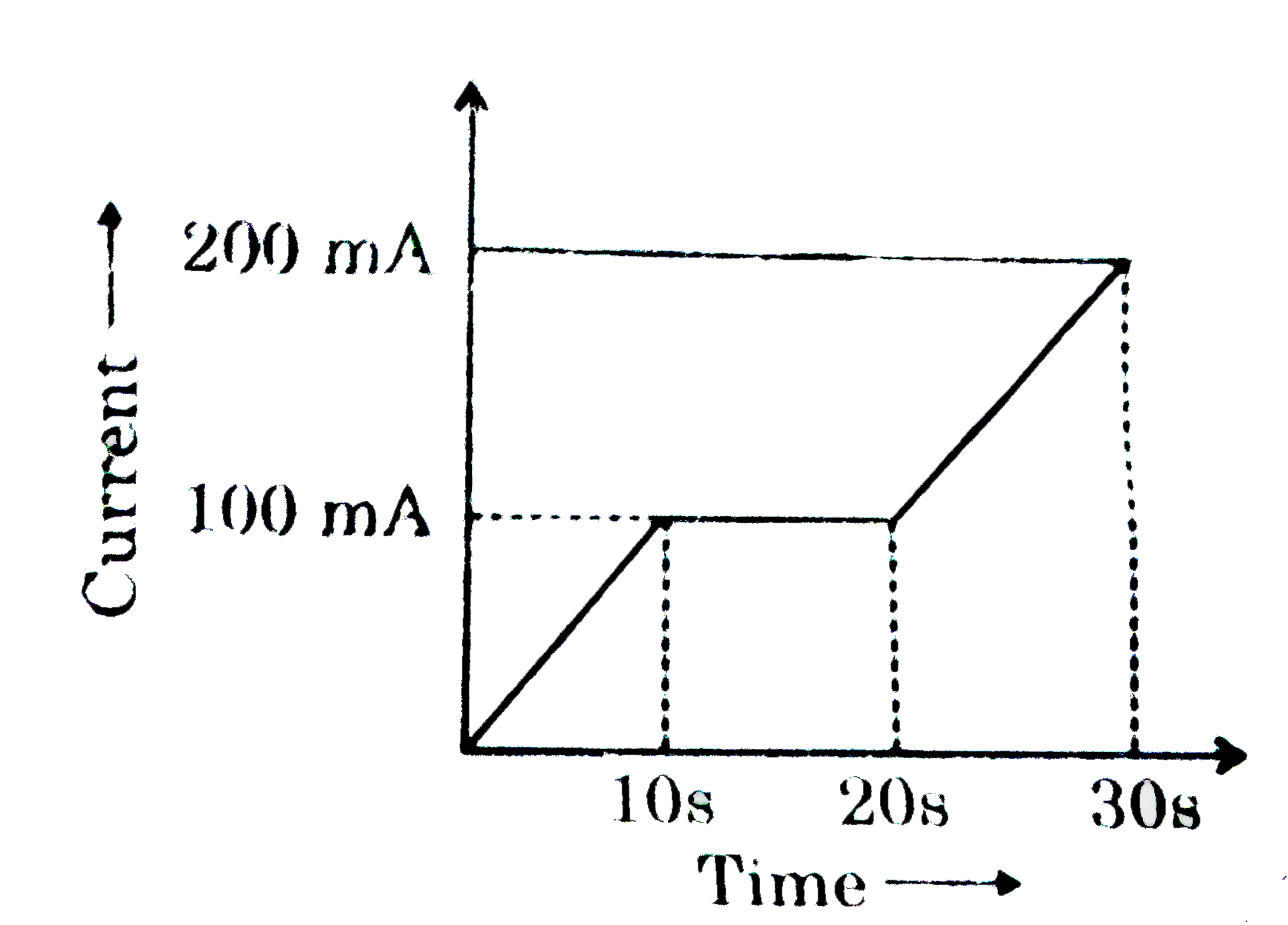 Am2Bm3Cm4Dm63.5
Am2Bm3Cm4Dm63.5In a Cu-voltameter, mass deposited in 30 s is m gm. If the time-current graph is shown in the following figure
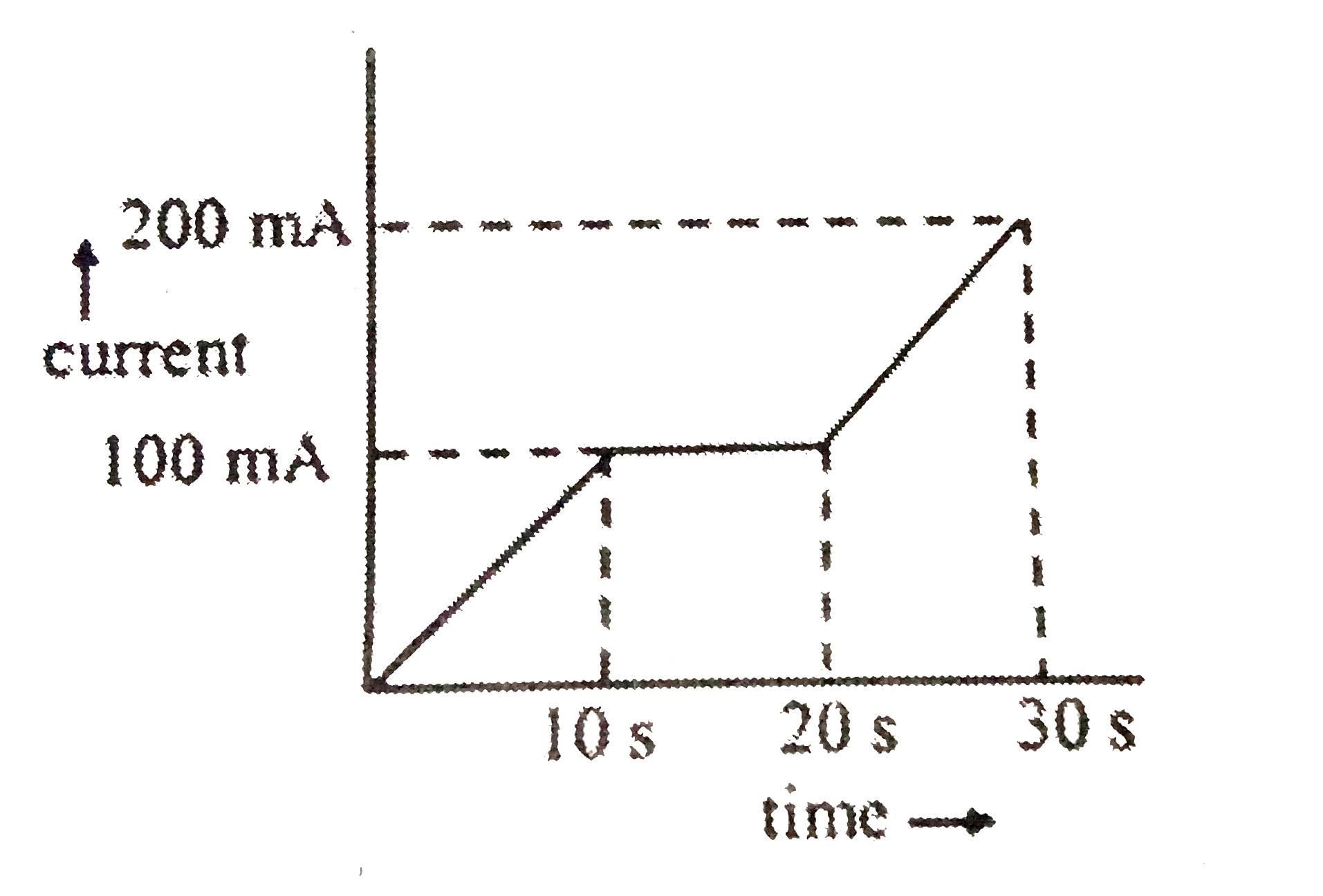
What is the electrochemical equivalent of Cu?Am/2Bm/3Cm/4Dm63.5In a copper voltameter, mass deposited in 6 minutes is m gram. If the current-time graph for the voltameter is as shown here, then the E.C.E of the copper is
 Am/5Bm/300C5mDm/18000
Am/5Bm/300C5mDm/18000
The atomic weight of silver and copper are 108 and 64. A silver voltameter and a copper voltameter are connected in series and when current is passed 10.8 gm of silver is deposited. The mass of copper deposited will be
View SolutionCurrent varies with time as shown in the graph

The current is
View SolutionIn a copper voltmeter, mass deposite in 30 seconds is 'm' gram. If the time-current graph is as shown in figure. ECE of copper is

View SolutionThe chemical equivalent of copper and zinc are 32 and 108 respectively. When copper and silver voltameter are connected in series and electric current is passed through for sometimes, 1.6 g of copper is deposited. Then, the mass of silver deposited will be
View SolutionCurrent varies with time as shown in the graph

This current is
View Solution
