Which is the strongest acid in the following ?
A
HCIO3
B
HCIO4
C
H2SO3
D
H2SO4
Video Solution
Text Solution
The correct Answer is:B
All are oxoacids. In oxoacids, the acidic strength is usually decided by the oxidation state of the central atom. Higher the oxidation state, greater the acidic strength:
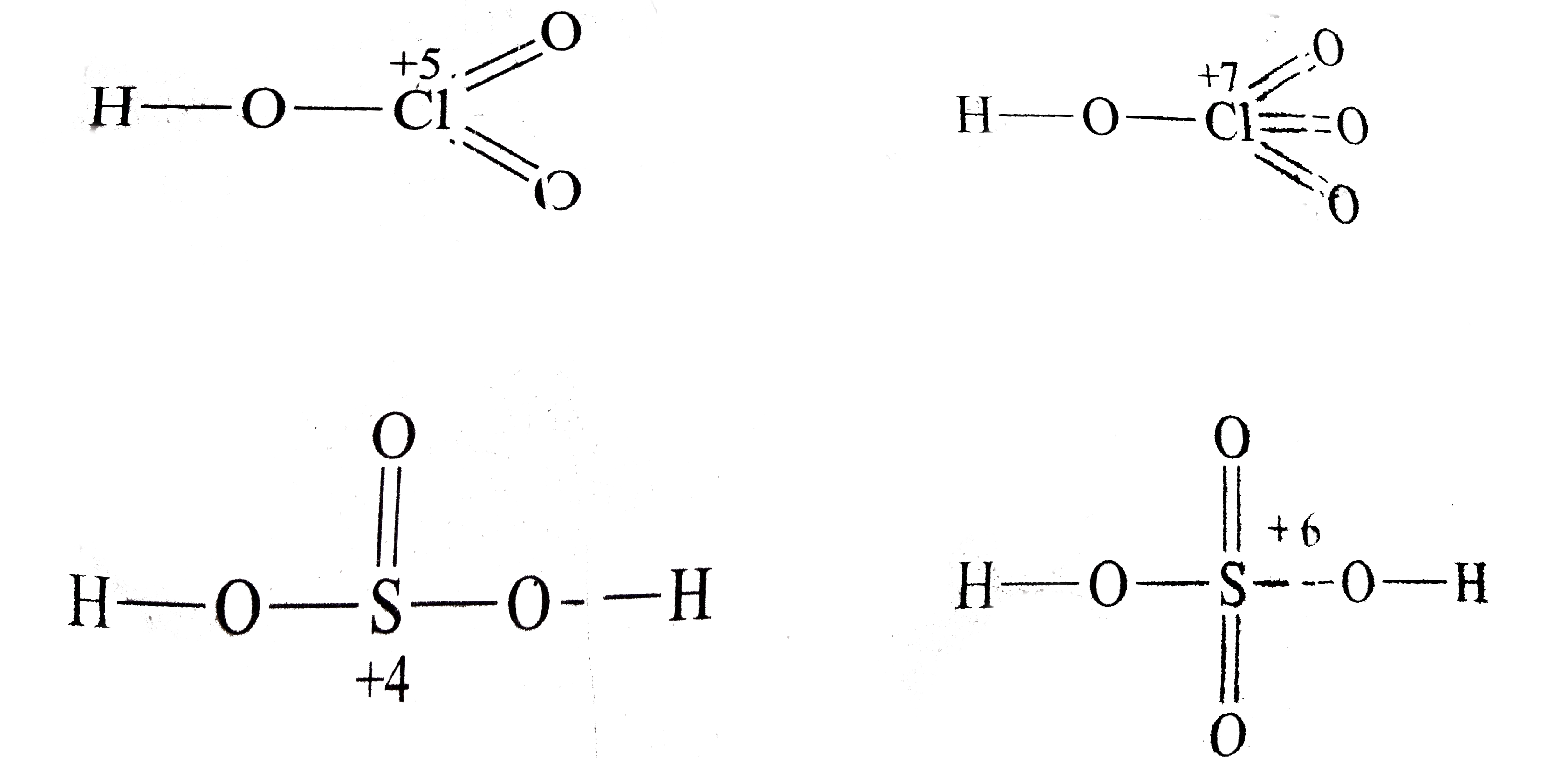
H−O−O∣∣S+4−O−H , H−O−O∣∣S∣∣O+6−O−H
Thus, the correct order of acidic strength is
+7+6+5+4>>>HClO4H2SO4HClO3H2SO3
|
Topper's Solved these Questions
- EQUILIBRIUMBOOK - R SHARMACHAPTER - EQUILIBRIUMEXERCISE - QUESTION BANK
26 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTSBOOK - R SHARMACHAPTER - CLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTSEXERCISE - ARCHIVES
37 Videos
- GENERAL ORGANIC CHEMISTRYBOOK - R SHARMACHAPTER - GENERAL ORGANIC CHEMISTRYEXERCISE - Archives
36 Videos
Similar Questions
Knowledge Check
Which is the strongest acid in the following ?
AH2SO4BHClO3CHClO4DH2SO3Which is strongest acidic?
ACH3CH2COOHBCH3COOHCC6H5COOHDC6H5CH2COOHWhich of the following strongest acid in the following?
AHCIO3BHCIO4CH2SO3DH2SO4
Which acid is the strongest ?
View SolutionWhich of the following is the strongest acid in water?
View SolutionWhich is the strongest conjugate acid ?
View SolutionWhich of the following is strongest acid among following
View SolutionFor the following compounds, which is the strongest base and which is strongest acid?
View Solution

