Which of the trichlorides is hydrolyzed most easily?
A
SbCl3
B
NCl3
C
AsCl3
D
PCl3
Video Solution
Text Solution
The correct Answer is:2
The ease of hydrolysis depends upon the tendency of the control atom to attract the lone pair of electrons from water which decreases as the electronegativity of the central atom(E) in ECl3 decreases down the group . Thus, the ease of hydrolysis of trichlorids decreases in the order:
NCl3>PCl3>AsCl3>SbCl3>BiCl3
|
Similar Questions
Knowledge Check
Which of the following chlorides hydrolyses most easily?
ACH3CH2CH2ClBCH2=CH=ClCCH2=CH−CH2 ClD(CH3)2CHCH2ClWhich of the following chlorides hydrolyses most easily
ACH3CH2CH2CIBCH2=CH−CICCH2=CH−CH2CID(CH3)2CHCH2CIWhich of the the following esters gets hydrolysed most easily under alkaline conditions?
A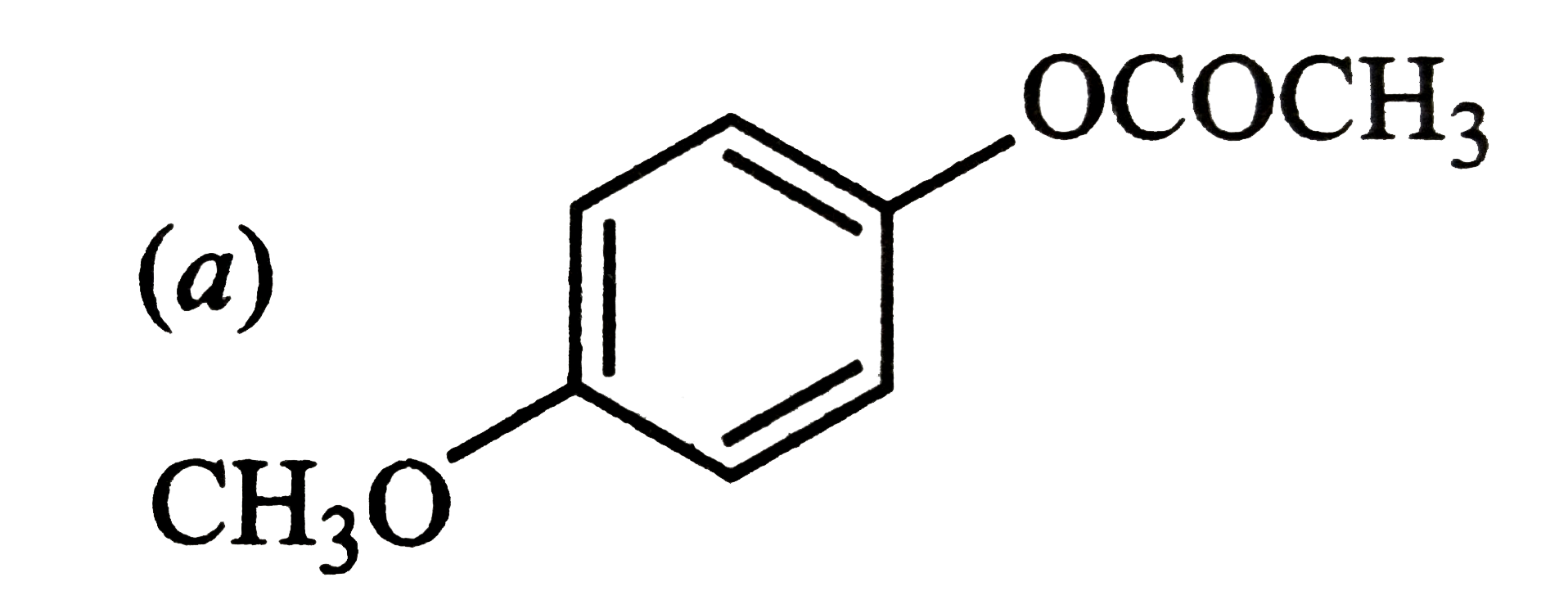 B
B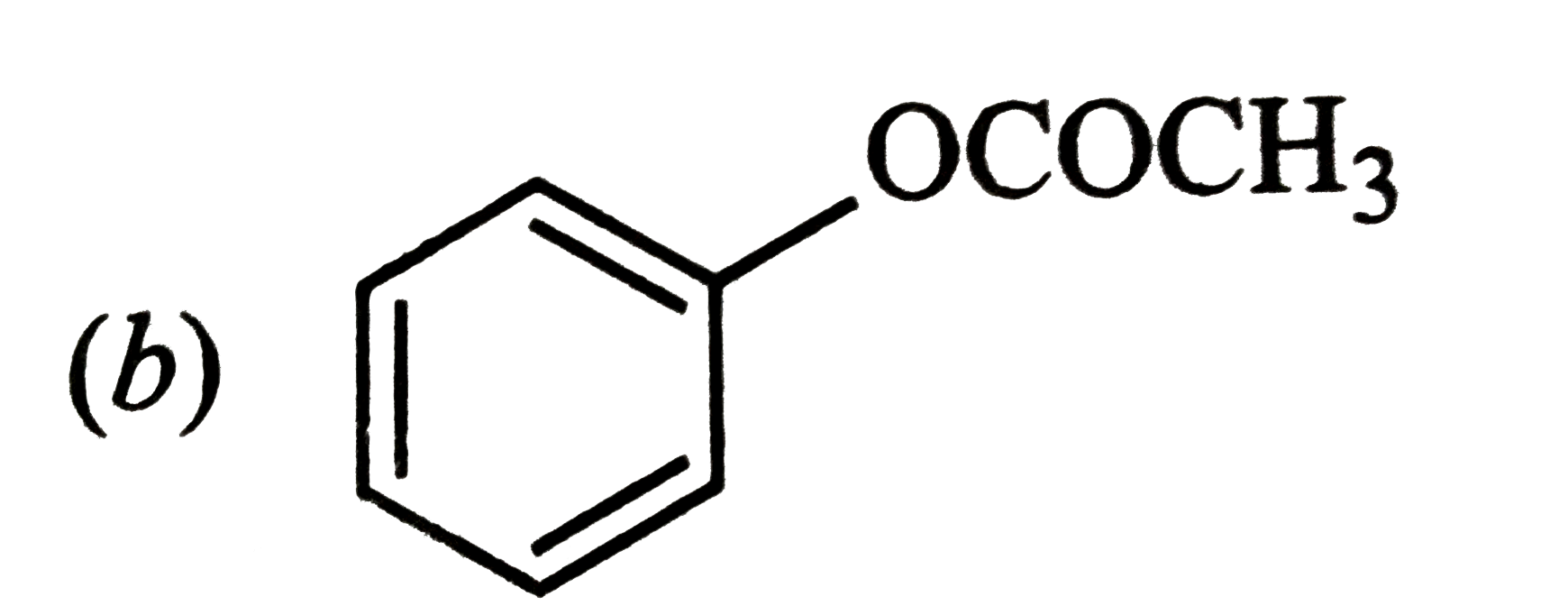 C
C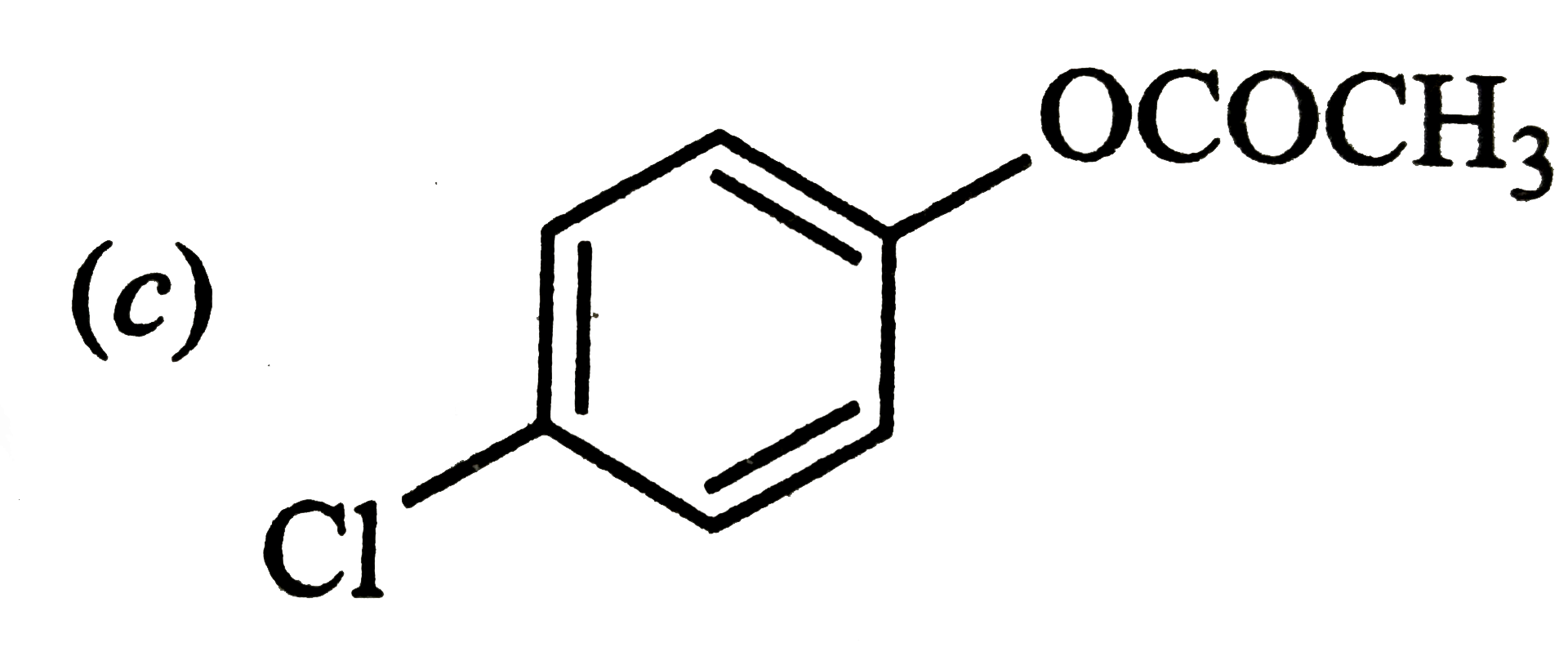 D
D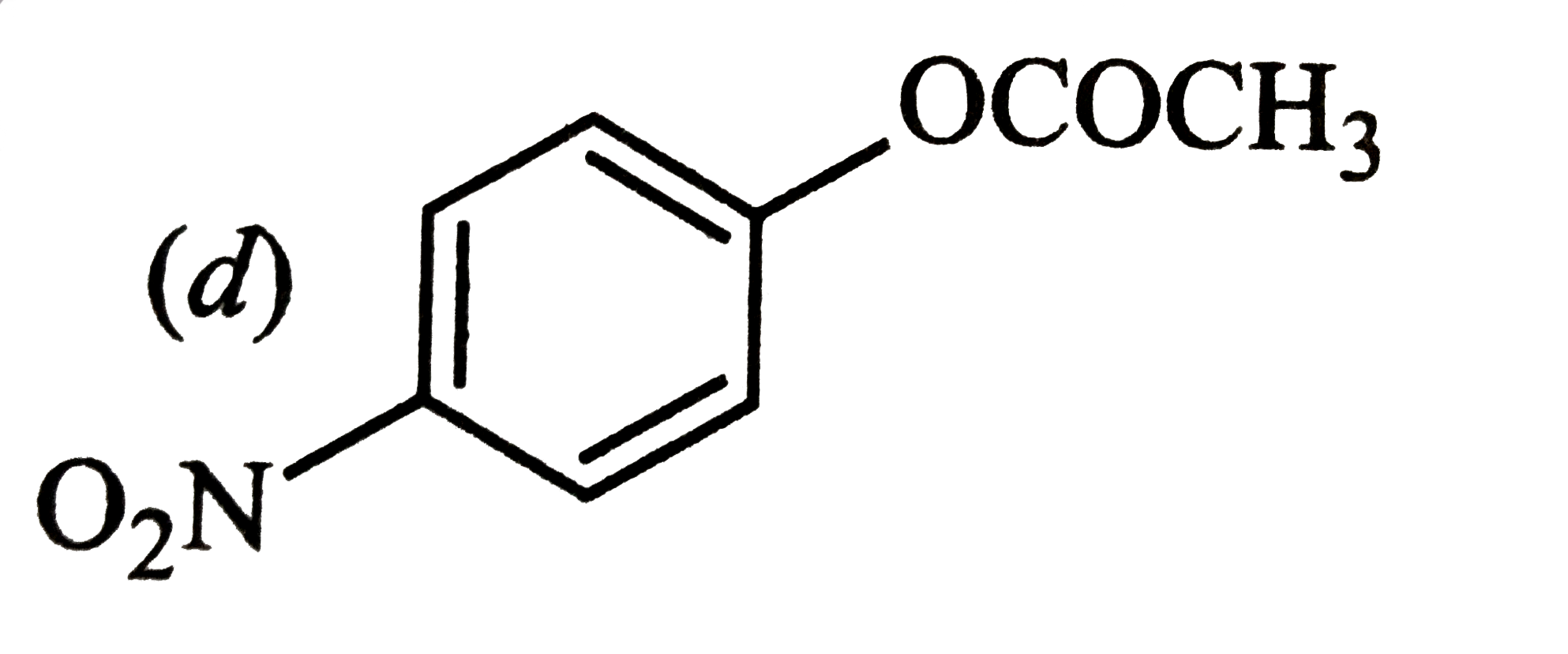
Which of the following decarboxylates most easily:-
View SolutionWhich of the following undergoes Hydrolysis most easily:
View SolutionWhich of the following undergoes Hydrolysis most easily:
View SolutionWhich of the following can decarboxylated most easily ?
View SolutionWhich of the following is reduced most easily to an alkane by NaBH4orLiAlH_(4)?
View Solution

