Which of the following compounds of xenon is nonpolar?
A
XeO3
B
XeO2F2
C
XeOF4
D
XeF4
Video Solution
Text Solution
The correct Answer is:4
It has zero dipole moment because it is square plannar:
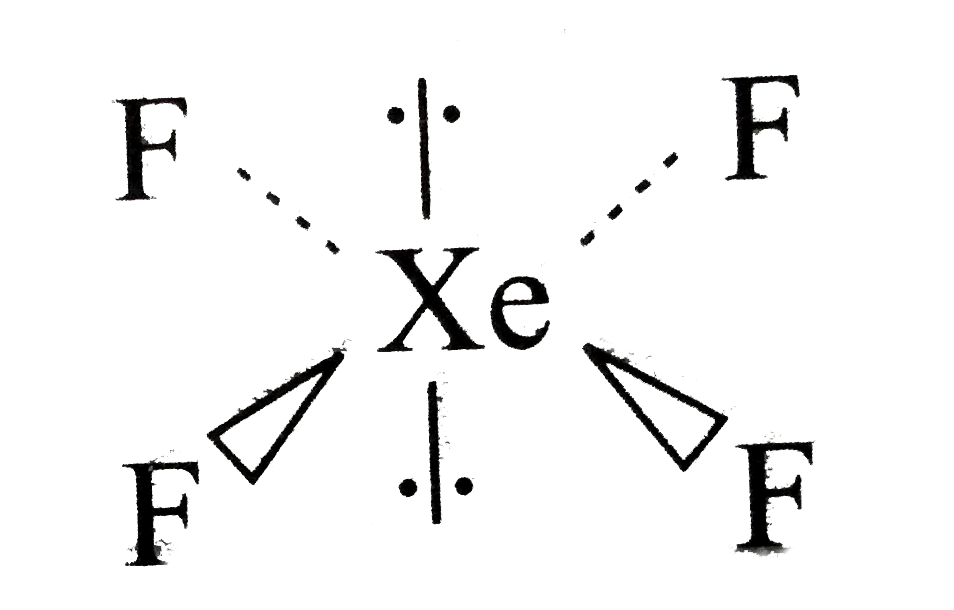
XeO3 contains three π−bonds, and the remaining four eletron pairs form a tetrahedron with one corner occupied by a lone pair of eletrons.
XeO2F2 Is trigonal bipyramidal with one of the equatorial positions occupied by a lone pair.XeOF4 is octahedral with one of the corners occupied by a lone pair.
|
Similar Questions
Knowledge Check
Which of the following compound cannot be prepared ?
AXeF3BXeF2CXeF4DXeF6Which of the following compound of xenon has pyramidal geometry ?
AXeOF4BXeF2CXeO3DXeF4Which of the following compound of xenon does not exists ?
AXeF6BXeF4CXeF5DXeF2
Which one of the following reactions of Xenon compound is not feasible ?
View SolutionWhich of the following reactions of xenon compounds in not feasible?
View SolutionWhich one of the following reactions of xenon compounds is not feasible ?
View SolutionWhich one of the following reactions of xenon compounds is not feasible ?
View SolutionWhich of the following Xenon compound does not produce explosive XeO3 on its complete hydrolysis?
View Solution
