Hydroxylamine (NH2OH) is manufacture in large quantities to make cycloexanone oxime, which is covnerted to caprolactam and then polymerized to give
A
Bakelite
B
Nylon-6
C
Nylon -6, 6
D
Melmac
Video Solution
Text Solution
The correct Answer is:2
|
Similar Questions
Knowledge Check
A carboxylic acid X is treated with bromine(1 mole) in the presence of a little phosphouus. The isolated product is heated with a strong base and then oxidized with O3/Zn−H2O. one of the two oxidation products is a netural compound which reacts with hydroxylamine to give an oxime, X can be
ACH3CH2CH(CH3)CH2CO2HBCH3CH2CH(CH3)CO2HCCH3CH2CO2HD(CH3)CHCH2CO2HOxides
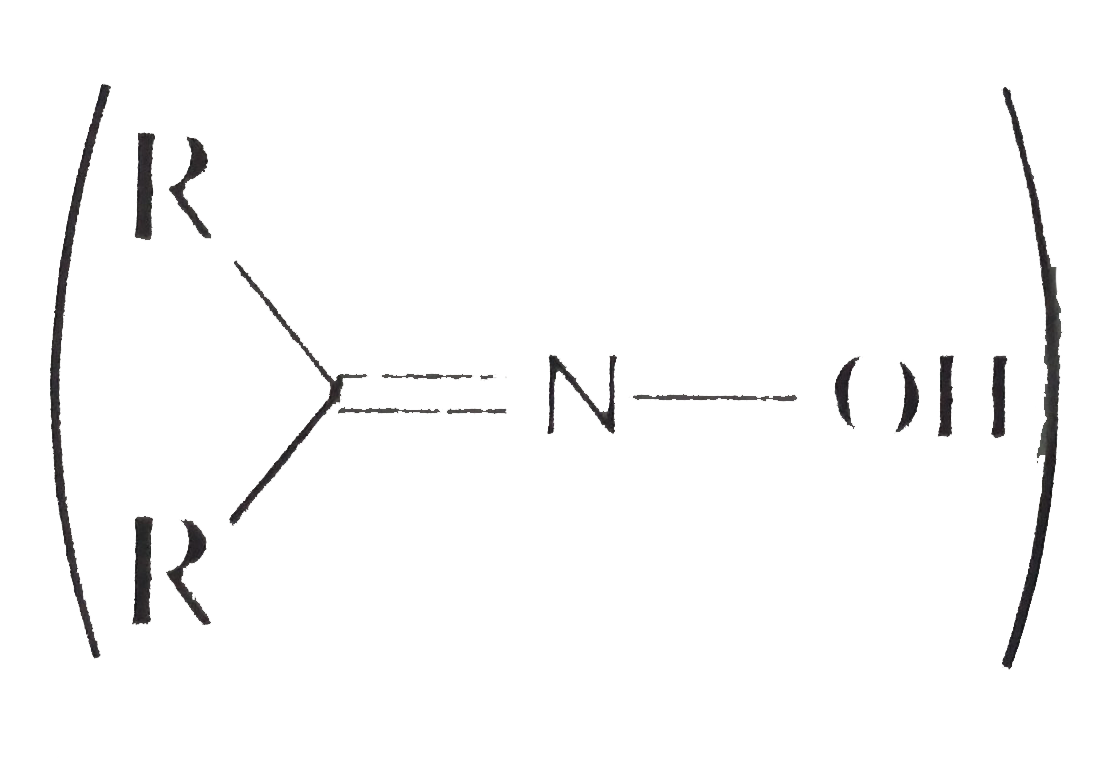 are more acidic than hydroxylamine (NH2OH).
are more acidic than hydroxylamine (NH2OH).
Conjugate base of oxime is resonance stabilised.AIf both (A) are (R) are true and (R) is the correct explanation of (A).BIf both (A) and (R) are true but (R) is not the correct explanation of (A).CIf (A) is true but (R) is false.DIf (A) is false but (R) is true.Acetaldehyde reacts with NH2OH to give
Aacetal amineBacetal oxideCacetaldoximeDamino acetal
Which of the following carbonyl compound will give two products after reaction with NH2OH:
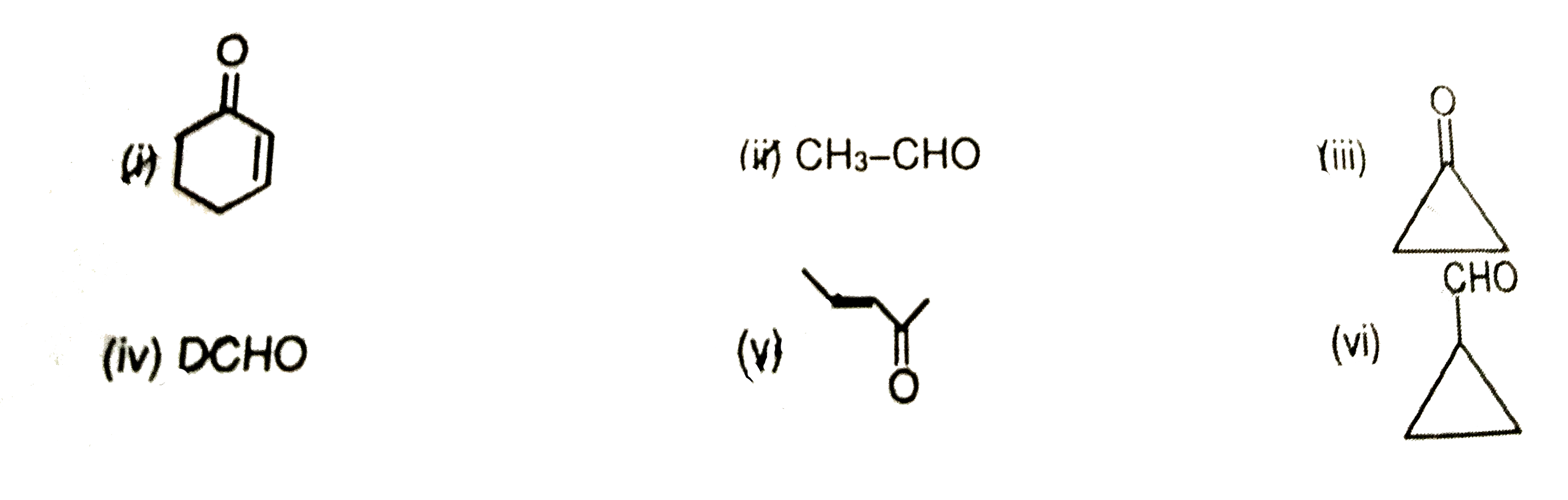
View SolutionHow many of the following carbonyl compound will give two products after reaction with NH2OH :
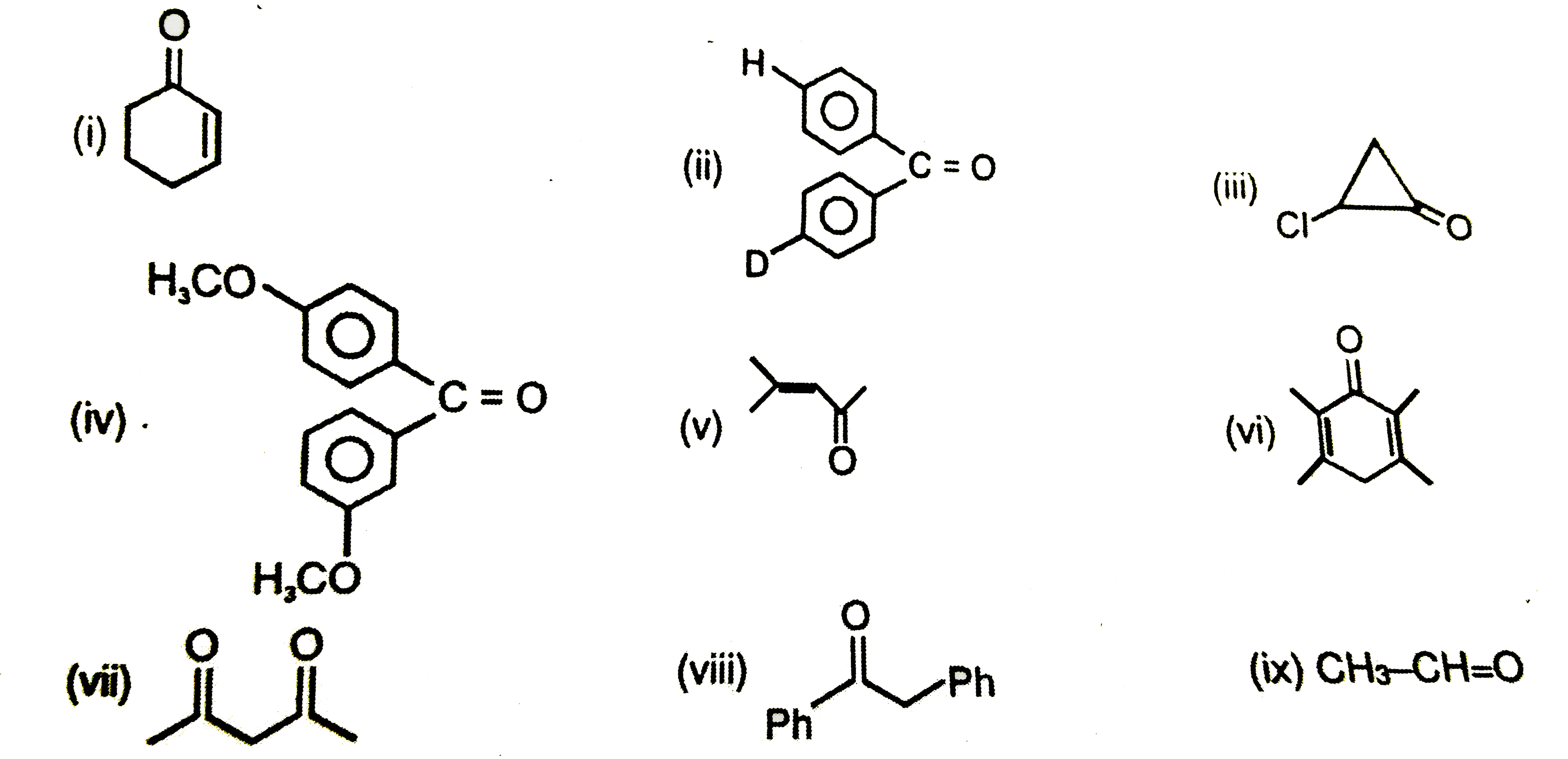
View SolutionAl2O3 formation from aluminium and oxygen involves evolution of a large quantity of heat, which makes aluminium use in :
View SolutionWhich will not give precipitate with NH4OH Solution ?
View SolutionWhich of the following will form only one oxime on reaction with NH2OH solution ?1
View Solution


