The product obtained a result of a reaction of nitrogen with CaC2 is
A
Ca2CN
B
CaCN2
C
CaCN
D
CaCN3
Video Solution
Text Solution
The correct Answer is:2
Calcium cyananmide [Ca2+N−=C=N−] is formed :
CaC2+N21273K−−−→CaCN2+C
since calcium cyanamide reacts with water to yield ammonia it is used as a fertilizer under the name nitrolim (CaCN2+C)
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
The product obtained a result of a reaction of nitrogen with CaC2 is
ACaCN3BCa2CNCCaCN2DCaCNThe product obtained as a result of a reaction of nitrogen with CaC2 is
ACa(CN)2BCaCNCCaCN3DCa2CNThe product obtained as a result of a reaction of nitrogen with CaC2 is
ACaCN3BCa2CNCCa(CN)2DCaCN
The product obtained a result of a reaction of nitrogen with CaC2 is
View SolutionThe product obtained a result of a reaction of nitrogen with CaC2 is
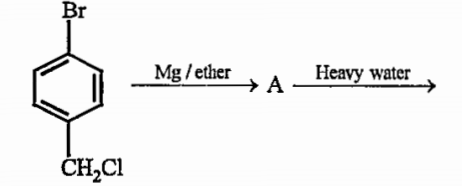
View SolutionThe product obtained from the reaction is:
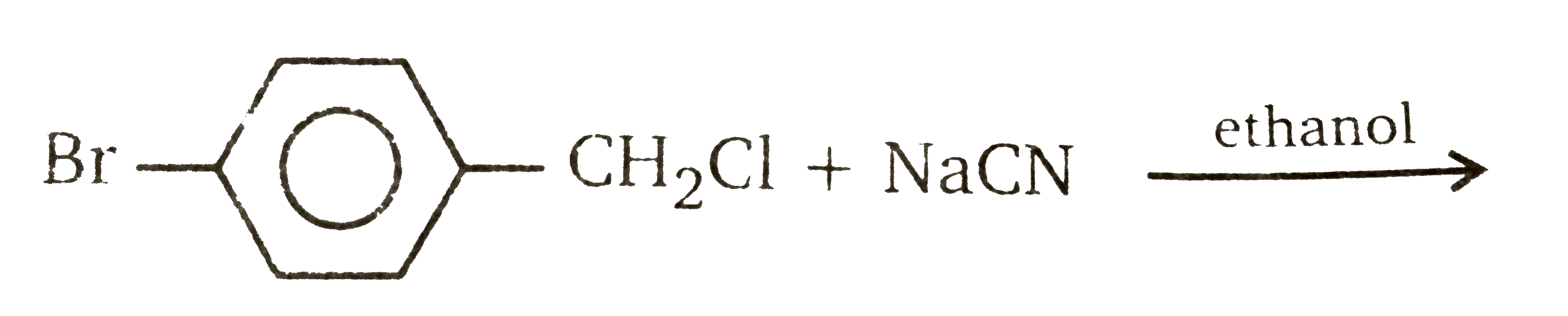
View SolutionThe major product obtained in the reaction :
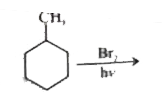
View SolutionThe major product obtained in the reaction :

View Solution