The major product (X) of the reaction
CH3CH2NH2+HCHO+HCOOHΔ−−→X is .
(CH3CH2)2NH
(CH3)2NH
CH3CH
A mixture of all the
The correct Answer is:C
To determine the major product X of the reaction between ethylamine (CH3CH2NH2), formaldehyde (HCHO), and formic acid (HCOOH) upon heating, we can follow these steps:
1. Identify Reactants: The reactants are ethylamine, formaldehyde, and formic acid. Ethylamine is a primary amine.
2. Understand the Reaction Type: The reaction involves a primary amine reacting with formaldehyde and formic acid. This is a type of methylation reaction where the amine is converted to a secondary amine.
3. Methylation Process: In this reaction, the primary amine (ethylamine) will react with formaldehyde to form an N-methylated product. The hydrogen atom from the amine group will be replaced by a methyl group from formaldehyde.
4. Formation of the Product: The reaction can be summarized as follows:
- Ethylamine (CH3CH2NH2) reacts with formaldehyde (HCHO) to form N-methyl-ethylamine (CH3CH2NHCH3).
- The formic acid can help stabilize the intermediate formed during the reaction.
5. Final Structure of Product X: The major product X after the reaction is N-methyl-ethylamine, which has the structure:
CH3CH2NHCH3
Conclusion:
The major product X of the reaction is N-methyl-ethylamine, represented as CH3CH2NHCH3.
- Ethylamine (CH3CH2NH2) reacts with formaldehyde (HCHO) to form N-methyl-ethylamine (CH3CH2NHCH3).
- The formic acid can help stabilize the intermediate formed during the reaction.
CH3CH2NHCH3
The major product X of the reaction is N-methyl-ethylamine, represented as CH3CH2NHCH3.
Similar Questions
Knowledge Check
The major product of the reaction
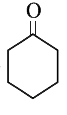 +(CH3)2NhNaBH3CN−−−−−−−−→CH3OH is A
+(CH3)2NhNaBH3CN−−−−−−−−→CH3OH is A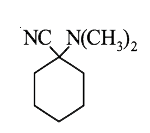 B
B C
C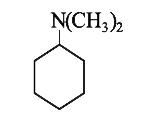 D
D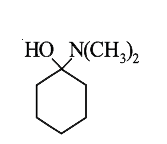
The major product formed in the reaction is :
_E01_202_Q01.png) +(CH3)2CHCH4BrAlCl3−−→ΔA
+(CH3)2CHCH4BrAlCl3−−→ΔA_E01_202_O01.png) B
B_E01_202_O02.png) C
C_E01_202_O03.png) D
D_E01_202_O04.png)
The major product formed in the following reaction is CH3CH(Cl)CH2−CH2OHaq.KOH−−−−→
ACH3CH=CH−CH2OHBCH2=CH−CH2−CH2OHCCH3−CH−CH2 | | O−CH2DCH3−C∣OHH−CH2−CH2OH
major product of the following reaction is : CH3CH=CHCO2CH3LiAIH4−−−→
View SolutionThe major product of the reaction between CH3CH2ONa and (CH3)3CCl in ethanol is
View Solution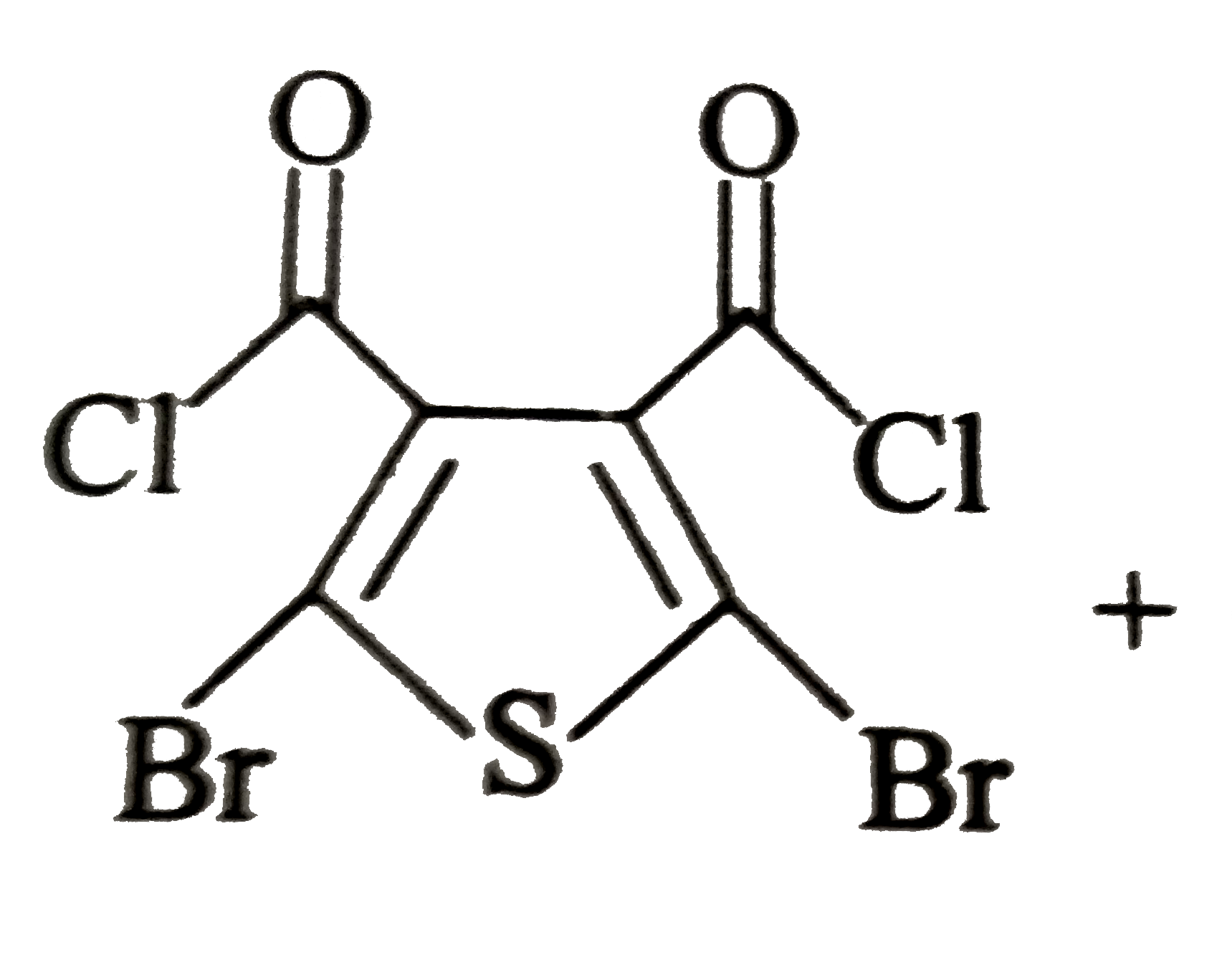 CH3CH2CH2CH2−NH2Δ−−→'X'
CH3CH2CH2CH2−NH2Δ−−→'X'
Identify structure of 'X' :
View SolutionThe products (A) and (B) of the reaction
CH3CH2NH2(CH3CO)2O−−−−−−−→heat .
View SolutionMajor alkene of the following reaction is
CH3−CCH2CH3 | Δ→. +N(CH3)OH−
View Solution

