Among the following the most basic is
A
C6H5NH2
B
(C6H5)2NH
C
C6H11NH2
D
NH3
Video Solution
Text Solution
The correct Answer is:C
The decreasing order of basicity is
Cyclohexy1 −NH2>NH3>C6H5NH2>(C6H5)2NH
Aromatic amines are much less basic than alkylamines because the electron density from the lone pair of electrons (on the N atom) is delocalized into the ring (mainly to the ortho and para positions)
An increase in the number of phenyls bonded to N increases delocalization and decreases basicity In cyclohexy 1−NH2
(C6H11NH2) the electron density is localized and enhanced further by the+ I effect of the ring and thus this amine is the most basic
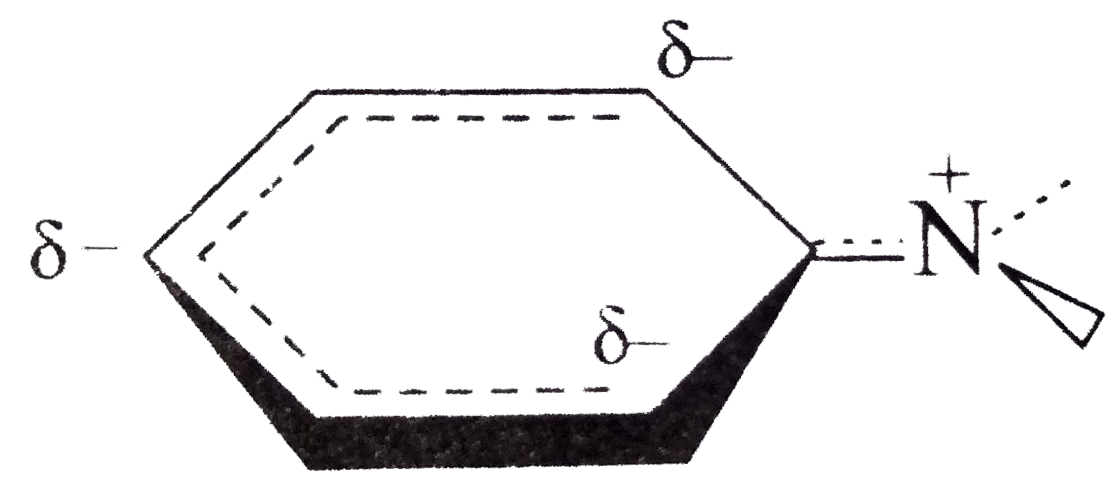 .
.
|
Similar Questions
Knowledge Check
Among the following, the most basic oxide is-
AAl2O3BSiO2CP2O5DNa2OAmongest the following the most basic in nature is
AbenzylamineBanilineCacetamideDp-nitroaniline.Amongst the following the most basic compound is :
ABenzylamineBAnilineCAcetanilideDp-Nitroaniline
Amongst the following the most basic compound is
View SolutionAmongest the following the most basic compounds is-
View SolutionAmongst the following the most basic compound is
View SolutionAmongst the following, the most basic compound is-
View SolutionAmongst the following the most basic compound is
View Solution

