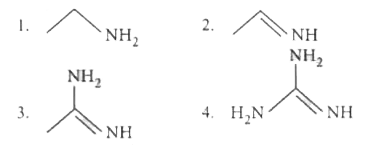
The correct order of the basicities of the following comounds is .
 .
.
A
A>B>C>D
B
C>A>B>D
C
B>A>C>D
D
A>C>B>D
Video Solution
Text Solution
The correct Answer is:D
Compound (A) is most basic because the resulting cation is resonance stabilized
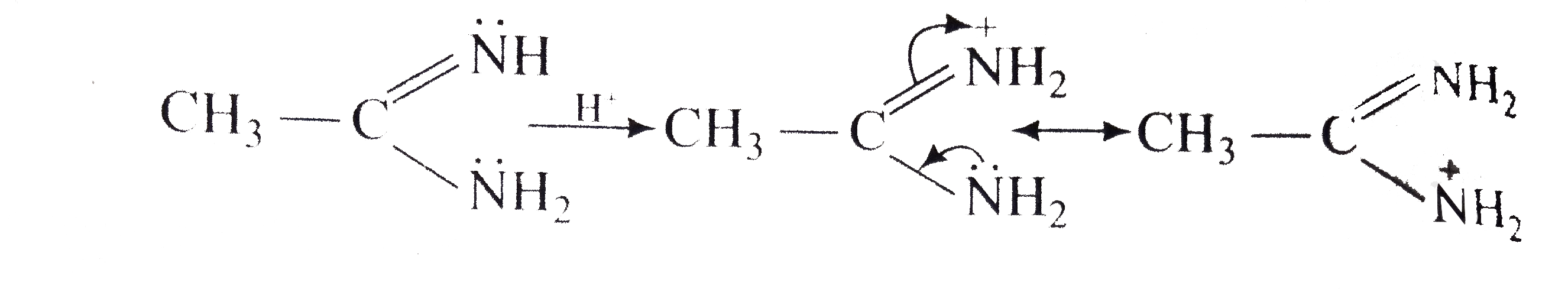
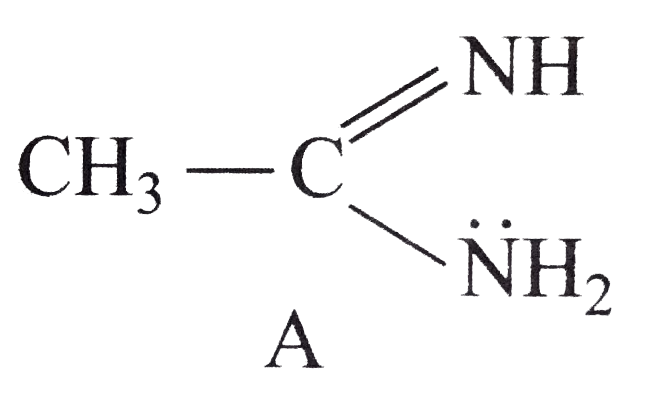
Moreover among all the compounds the imino N carries negative charge on account of resonance This facilitates protonation
Amides are far less basic than amines on account of resonance as well as inductive effects
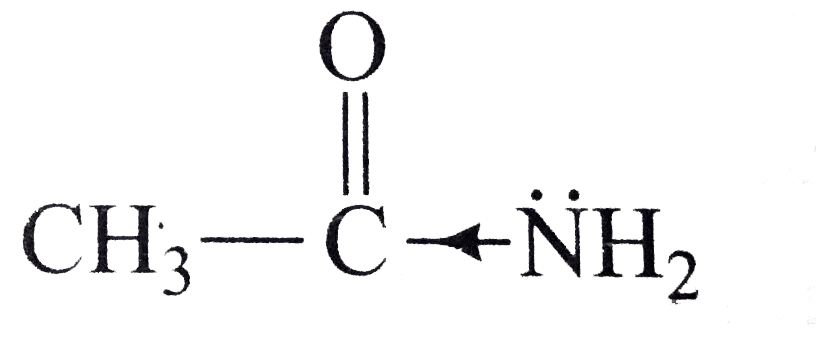 Due to the powerful electron-withdrawing effect of the carbony1 group the electron density near the nitrogen atom is quite less
Due to the powerful electron-withdrawing effect of the carbony1 group the electron density near the nitrogen atom is quite less
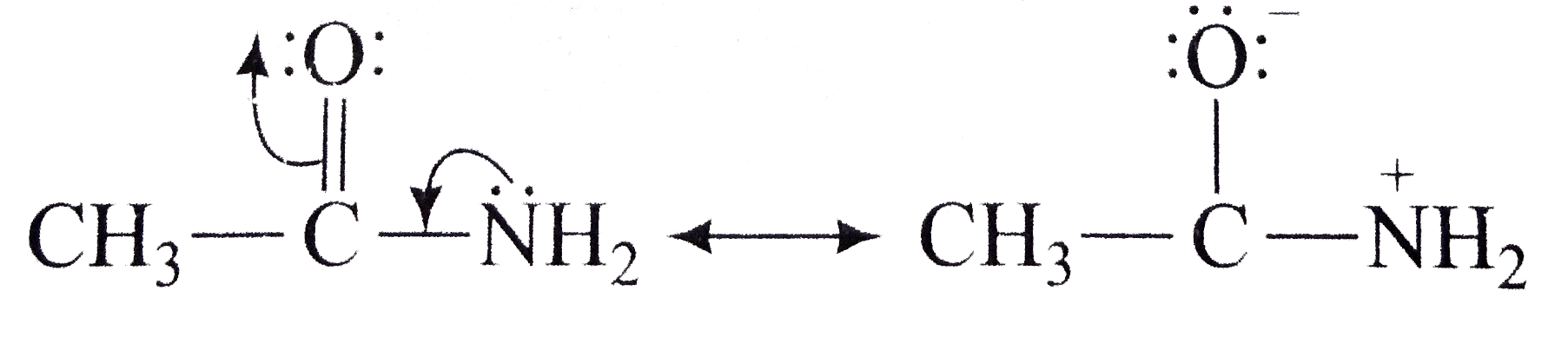
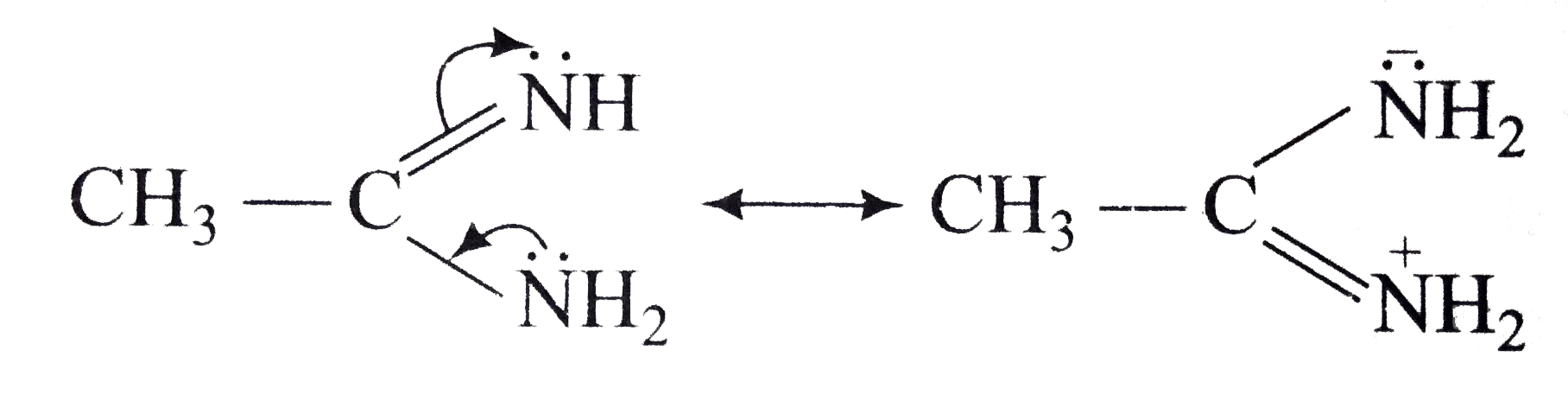
The lone pair on N atom is delocalized due to resonance Due to positive inductive effect of two alky1 groups C is more basic than B .
|
Similar Questions
Knowledge Check
The correct order of basicities of the following compounds is
 A2>1>3>4B1>3>2>4C3>1>2>4D1>2>3>4.
A2>1>3>4B1>3>2>4C3>1>2>4D1>2>3>4.the correct order of basicities of the following compounds is
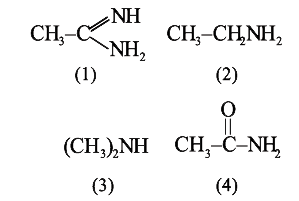 A(2)>(1)>(3)>(4)B(1)>(3)>(2)>(4)C(3)>(1)>(2)>(4)D(1)>(2)>(3)>(4)
A(2)>(1)>(3)>(4)B(1)>(3)>(2)>(4)C(3)>(1)>(2)>(4)D(1)>(2)>(3)>(4)The correct order of basicities of the following compounds is
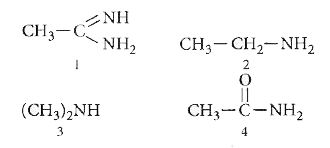 A2>1>3>4B1>3>2>4C3>1>2>4D1>2>3>4
A2>1>3>4B1>3>2>4C3>1>2>4D1>2>3>4
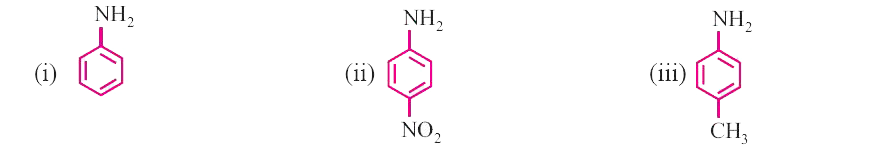
The correct order of basicities of following compounds is :
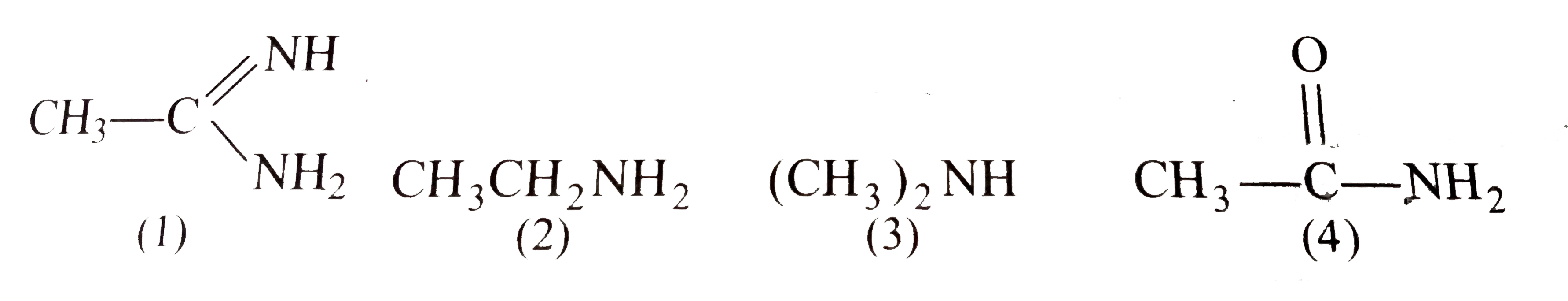
View SolutionThe correct order of basic strength for the following compounds is :
View SolutionThe correct order of increasing basicity of the following compound is

View SolutionThe correct order of basicity of the following species is

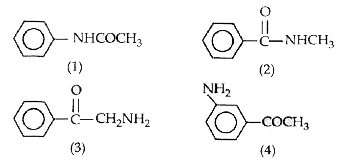
View SolutionThe correct order of basic strength of the following are
View Solution