Nitration of aniline is achieved by
A
direct nitration with nitrating mixture under reflux
B
using fuming HNO3
C
acetylation followed by nitration and subsequent hydrolysis
D
KNO3 and conc HNO3
Video Solution
Text Solution
The correct Answer is:D
Direct nitration of aniline should be avoided as it yields tarry oxidation products in addition to the nitro derivatives However we can control the nitration reaction by protecting the −NH2 group by acetylation reaction with acetic anhydride
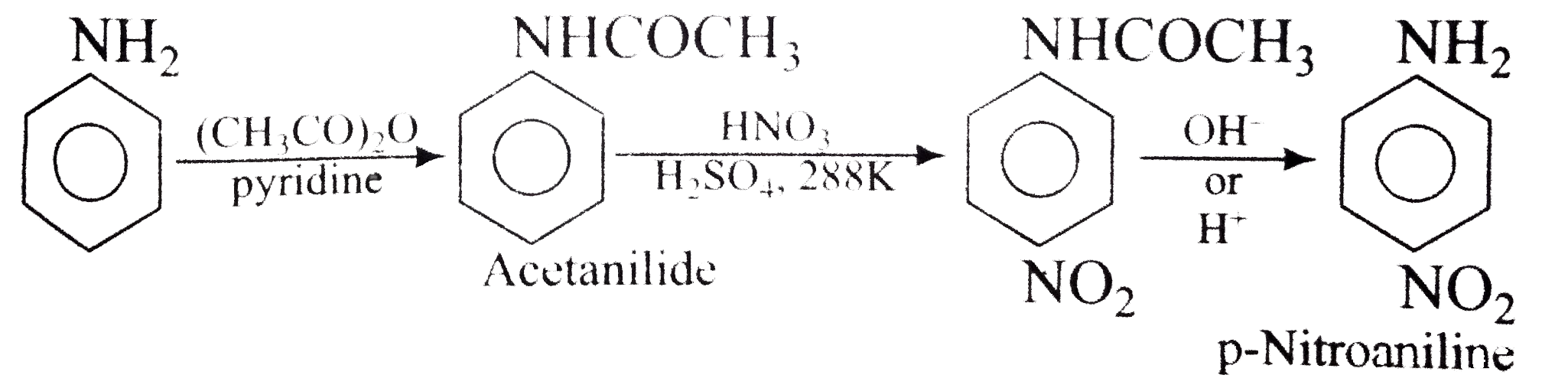 .
.
|
Similar Questions
Knowledge Check
Nitration of aniline is carried out after acylation because
AAcylation deactivates the −NH2 groupBOxidation can be preventedCo− " and " p- products are obtained in good yieldsDall the threeNitration of aniline in strong acidic medium also gives m-nitroaniline because
Ain absence of substituents nitro group always goes to m-positionBin electrophilic substitution reactions amino group is meta directiveCin spite of substituents nitro group always goes to only m-positionDin acidic (strong) medium aniline is present as anilinium ionNitration of aniline in strong acidic medium also gives m-nitroaniline because
Ainspite of substituents nitro group always goes to only m-positionBin electrophilic substitution reactions amino group is meta directiveCin absence of substituents nitro group always goes to m-positionDin acidic (strong) medium aniline is present as anilinium ion.
Direct nitration of aniline is not carried out. Explain.
View SolutionNitration of aniline in strong acidic medium also gives m-nitroaniline because
View SolutionNitration of aniline in strong acidic medium also gives m-nitroaniline because
View SolutionNitration of aniline also gives m - nitroaniline along with o - nitro and p - nitroaniline , in strong acidic medium because
View SolutionStatement 1: Nitration of aniline is generally performed by protecting amino group through acetylation and
Statement 2: Acetylation decreases the electron density in aromatic ring.
View Solution
