Diazonium salts form phenylhydrazines when reduced with
zinc and hydrochloric acid
(ii) stannous chloride and hydrochloric acid
(iii) sodium sulphite .
A
(i),(ii),(iii)
B
(ii),(iii)
C
(i),(ii)
D
(i),(ii)
Video Solution
Text Solution
The correct Answer is:B
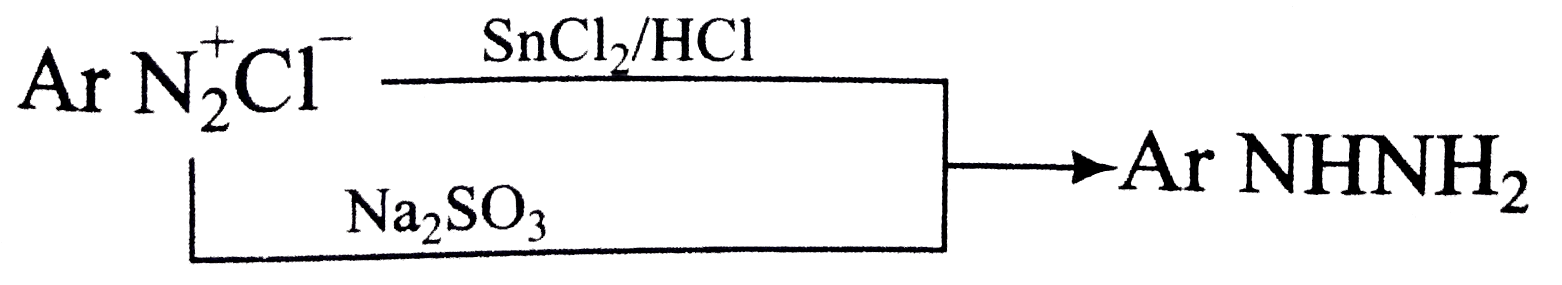 The reduction with sodium sulphite is carried out commercially if vigorous reducing agent agents like zinc and hydrochloric acid are used then the product is an aromatic amine
The reduction with sodium sulphite is carried out commercially if vigorous reducing agent agents like zinc and hydrochloric acid are used then the product is an aromatic amine
ArN_(2)^(+)Cr^(-) overset (Zn//HCI)rarr[ArNHNH_(2)]overset(ZnHC)rarrArNH_(2)+NH_(3) .
|
Similar Questions
Knowledge Check
Which one of the following is reduced with zinc and hydrochloric acid to give the corresponding hydrocarbon?
AEthyl acetateBAcetic acidCAcetamideDButan - 2 - oneWhich one of the following is reduced with zinc and hydrochloric acid to give the corresponding hydrocarbon?
Aethyl acetateBacetic acidCacetamideDbutan-2-oneWhich one of the following is reduced with zinc and hydrochloric acid to give the corresponding hydrocarbon ?
AEthyl acetateBButan-2-oneCAcetamideDAcetic acid
Zinc is treated with dilute hydrochloric acid.
View SolutionZinc oxide is dissolved in dilute hydrochloric acid,
View SolutionWhich one of the following is reduced with zinc and hydrochloric acid to give the corresponding hydrocarbon ?
View SolutionWhich one of the following is reduced with zine and hydrochloric acid to give the corresponding hydrocarbon?
View SolutionNitrobenzene when reacted with tin and hydrochloric acid, i.e., in acidic medium, the product formed is:
View Solution
