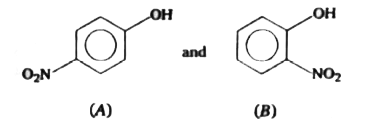
Out of the two compounds shown below, the vapour pressure of B at a particular temperature is expected to be :
A
higher than that of A
B
lower than that of A
C
same as that of A
D
can be higher or lower depending upon the size of the vessel
Video Solution
Text Solution
The correct Answer is:A
Compounds (B) exhibits intramolecular H-bonding has more vapour pressure than compound (A) involved in intermolecular H-bonding.
|
Similar Questions
Knowledge Check
Out of the two compound's shown below, the vapour pressure of B at a particular temperature is expected to be.
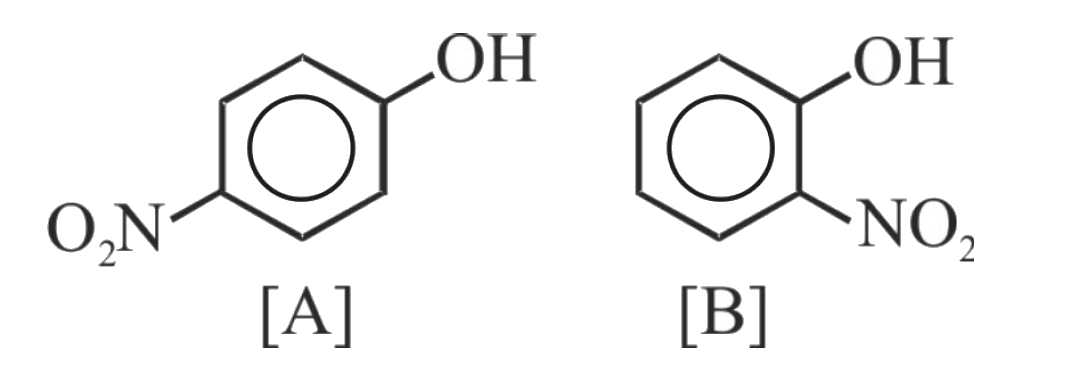 Ahigher than that of ABlower than that of ACsame as that of ADcan be higher or lower depending upon the size of the vessel
Ahigher than that of ABlower than that of ACsame as that of ADcan be higher or lower depending upon the size of the vesselOut of the two compounds shown below the vapour pressure of II at a particular temperature is expected to be
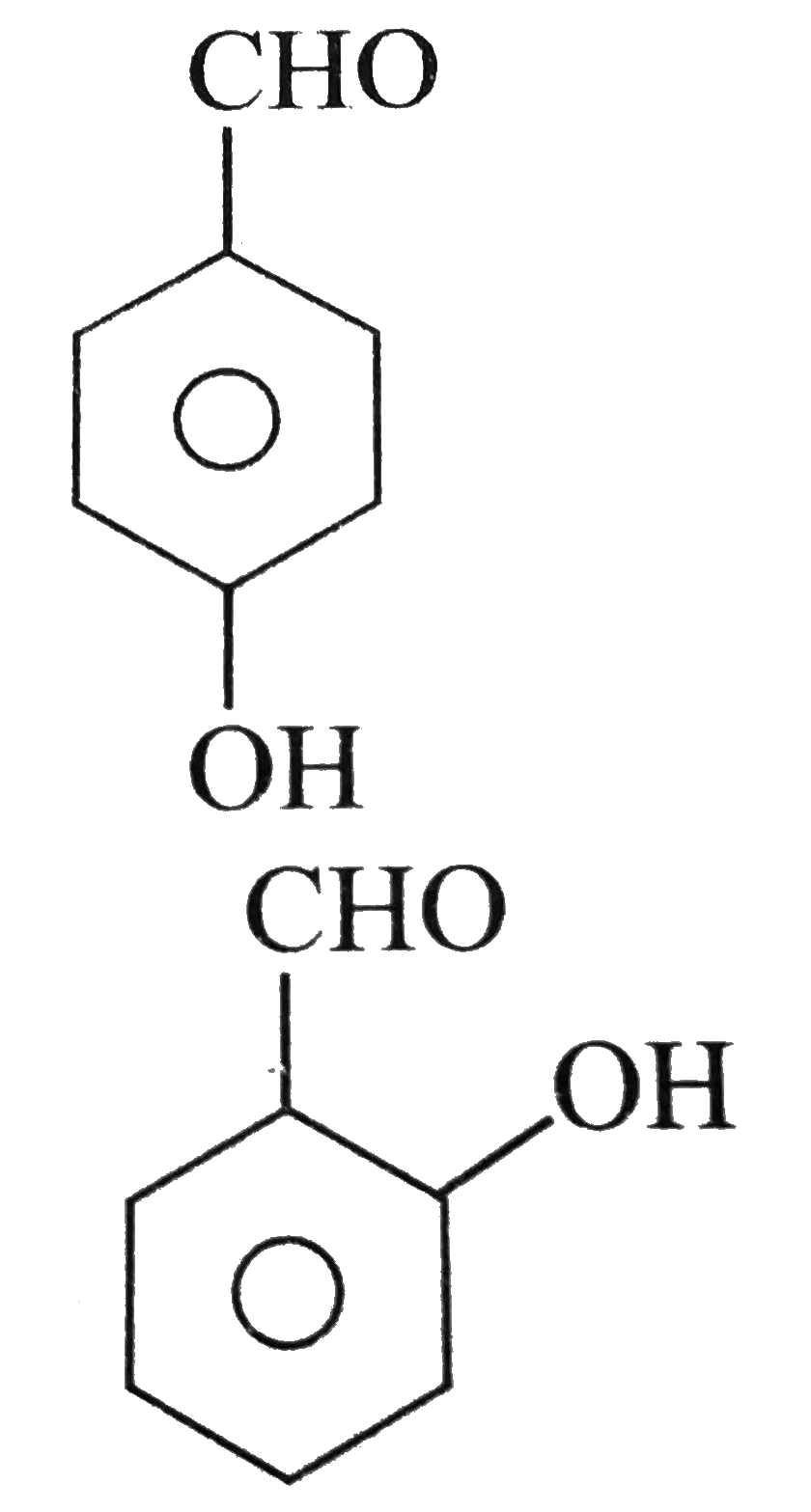 .AHigher than that of IBLower than that of ICSame as that of IDCan be higher or lower depending upon the size of vessel .
.AHigher than that of IBLower than that of ICSame as that of IDCan be higher or lower depending upon the size of vessel .Of the two compounds shown below , the vapour pressure of B at a particular temperature is
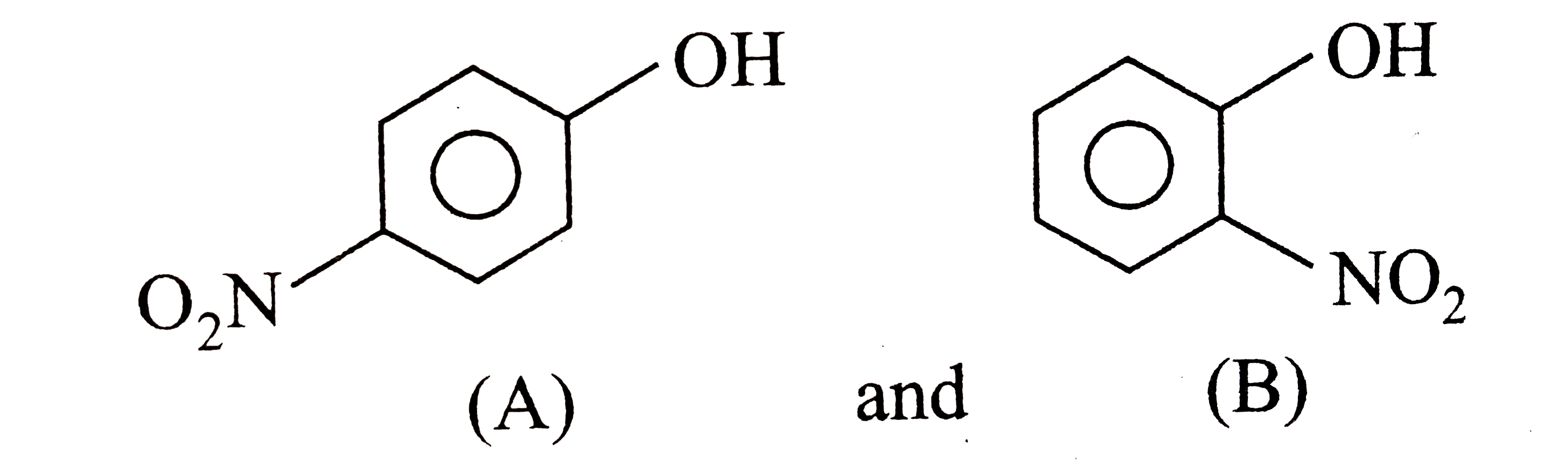 Ahigher than that of ABlower than that of ACsame as that of ADcan be higher or lower depending upon the size of the vessel
Ahigher than that of ABlower than that of ACsame as that of ADcan be higher or lower depending upon the size of the vessel
The vapour pressure of pure benzene is 22mm and that of pure toluene is 75mm at 20∘C. What is the composition of the solution of these two components that has a vapour pressure of 50mm at this temperature ? What is the composition of vapour in equilibrium with this solution ?
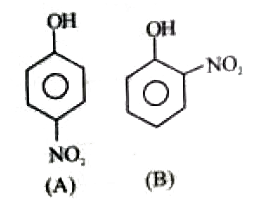
View SolutionOut of the compounds given below , the vapour presssure of (B) at a particular temperature is :
View SolutionThe two compounds shown below are :
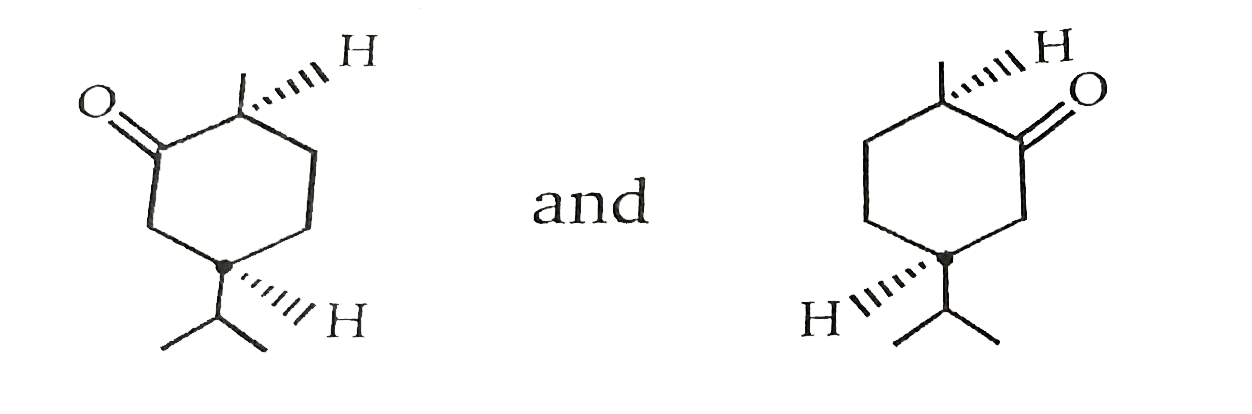
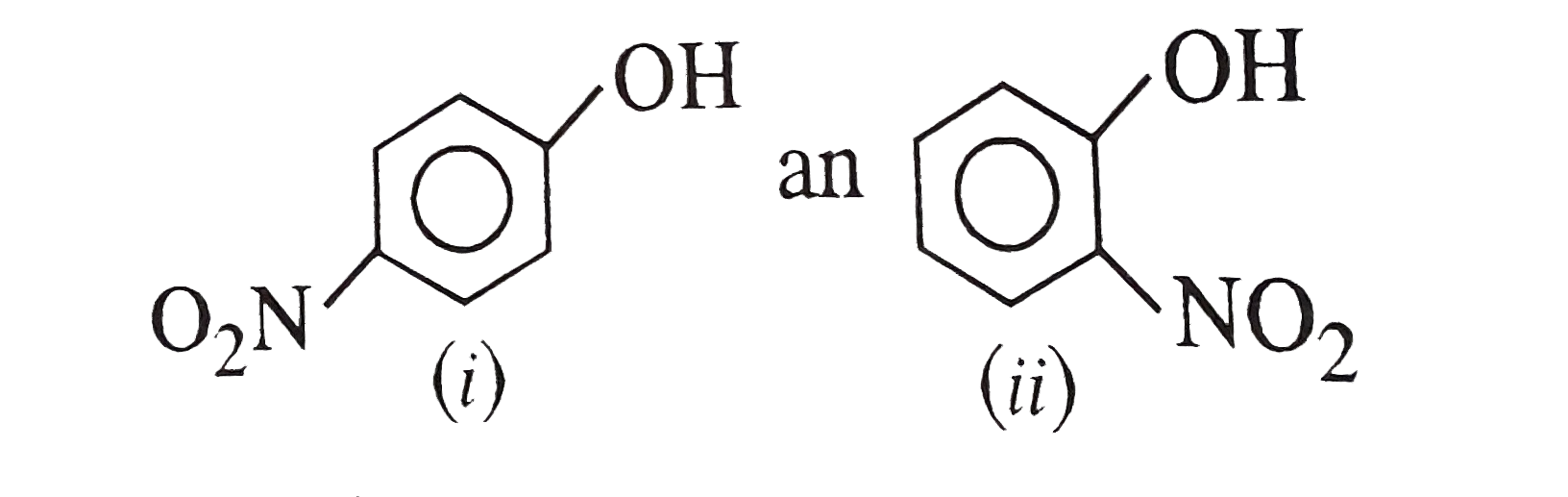
View SolutionFor the two compounds, the vapour pressure of (i) at a particular temperature is expected to be
View SolutionConsider the following compounds
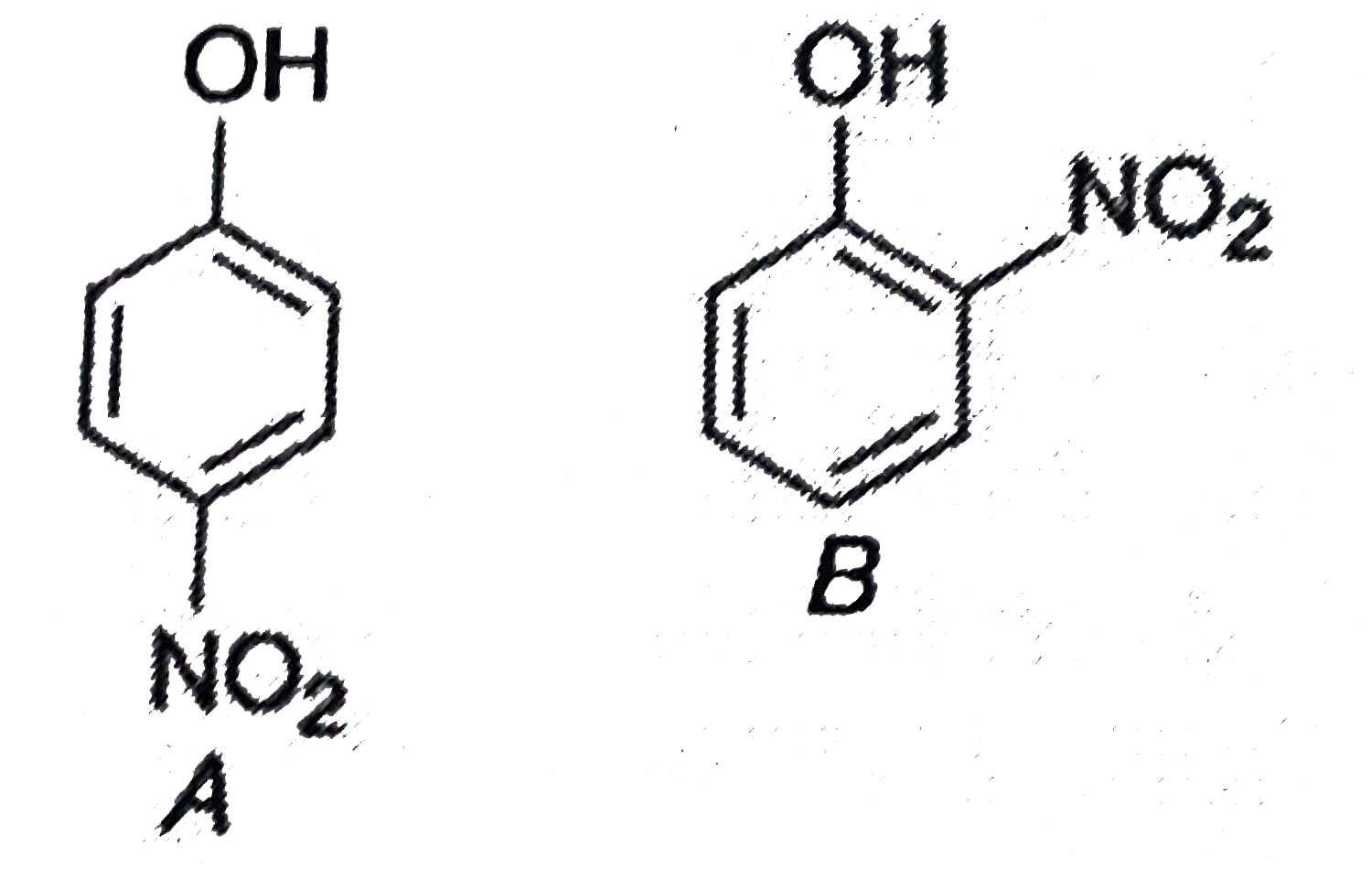
Among the compounds A and B, the vapour pressure of B at a particular temperature is
View Solution