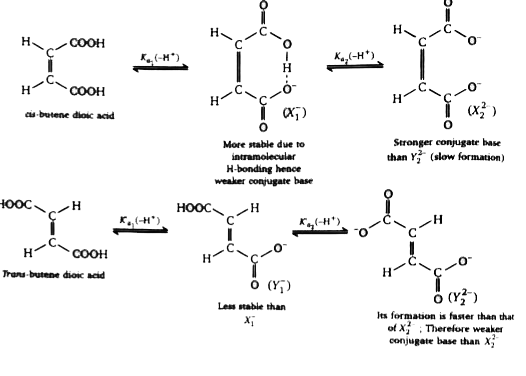
Cis butene dioic acid Ka1(−H+)−−−−−−−−−→←−−−−−−−−−X−1Ka2(−H+)−−−−−−−→←−−−−−−→X−2
Trans-butene dioic acid Ka1(−H+)−−−−−−−−−→←−−−−−−−−−Y−1Ka2(−H+)−−−−−−−→←−−−−−−→Y−2
The incorrect statement regarding above information is:
A
X2−2 species is more basic than Y2−2 species
B
X−1 species is more basic than Y−1 species
C
Ka1 is greater than K'a1
D
Ka2 is greater than K'a2
Video Solution
Text Solution
The correct Answer is:B
|
Similar Questions
Knowledge Check
R−O∣∣C−OHH3O+←−−−X[H]−−→RCH2NH2X is ,
AisonitriteBnitrileCnitriteDoximeRCOOHH3O+←−−−X4[H]−−→RCH2NH2
AAlkaneBAlkyl isonitrileCAldoximeDAlkane nitrileIf N2+3H2K⇐⇒2NH3 then 2N2+6H2K⇐⇒4NH3−K' is equal to
AK2B(K)1/3C1/√KD1/K2
Which H atom in the following ester is most acidic ?
1CH3−2CH2−O∣∣C−3CH2−O∣∣C−O−4CH25CH3
View Solution(A) cis-2-butene HCO3H−−−−→I
(B) trans-2-butene HCO3H−−−−→II
Correct statements are
View SolutionE+D⏐⏐↓H2O←−−BP←−−CaN2−−→AH2O−−→C⏐⏐↓+E
Which of the following characteristic is same for gases C and D?
View SolutionC2H5−O∣∣C−CICh2N2/Ag2∅H2O−−−−−−−−−−→AB2H6−−→B. A and B are
View SolutionY200∘C←−−−CaSO4.2H2O120∘C−−−→X.
X and Y are respectively:-
View Solution