If P to T are second period p-block elements then which of the following graph show corrrect relation between valence electrons in P2→T2
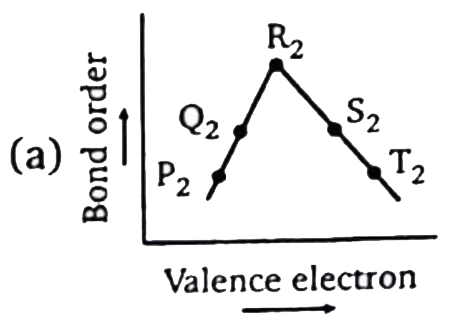
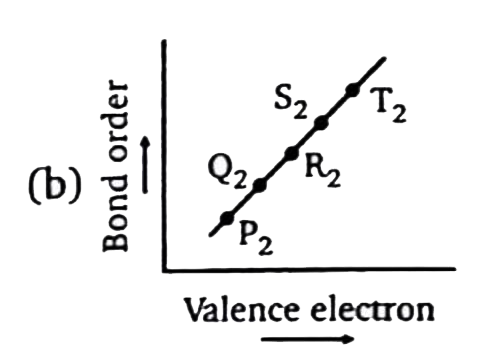
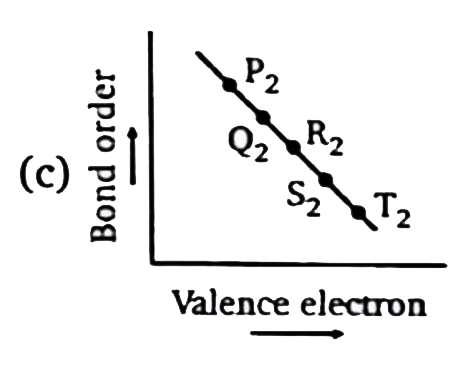
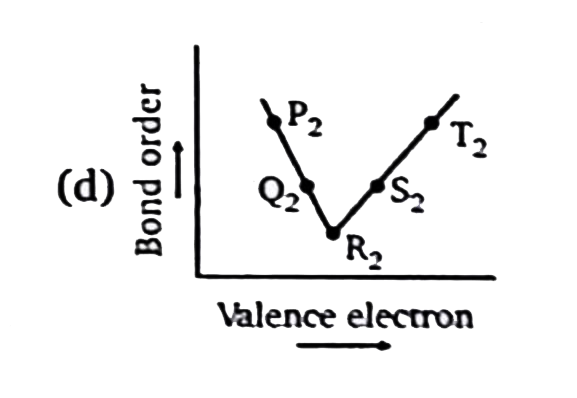
The correct Answer is:A
Topper's Solved these Questions
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWALChapter:CHEMICAL BONDING (ADVANCED)Exercise:Level 3
Explore 92 Videos
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWALChapter:CHEMICAL BONDING (ADVANCED)Exercise:ONE OR MORE ANSWER IS/ARE CORRECT
Explore 84 Videos
- CHEMICAL BONDING (ADVANCED)Book:VK JAISWALChapter:CHEMICAL BONDING (ADVANCED)Exercise:SUBJECTIVE PROBLEMS
Explore 72 Videos
- CHEMICAL BONDING (BASIC)Book:VK JAISWALChapter:CHEMICAL BONDING (BASIC)Exercise:Level 3 (Passive 11)
Explore 6 Videos
Similar Questions
Colour of compounds occurs due to phenomenon of polarisation, charge transfer, d-d transition and H.O.M.O.L.U.M.O transition. Most of the transition metal transition metal complex compound are coloured either due to d-d electrons transition or charge transfer and ionic compounds are coloured due to polarisation of anion. Q. Which of the following is correct about KFeII[FeIII(CN6)]andKFeIII[FeII(CN6)] complex compounds?
View SolutionWhich is correct statement ?
View SolutionThere are two groups of compounds A and B. Groups A contains three compounds Px4,Qy3,Rz2. Groups B also contains three compounds Sx4,Ty3,Uz2. Hybridization of each central atom of group A compounds is same as that of iodine in IBrCl− while in group B compounds it is same as that of iodine IBrCl+. Substituents X, Y and Z exhibit covalency of one in ground state. Then find the value of x/y.
Where, x and y are total number of lone pair present at central atoms of compounds of group A and B respectively.
View SolutionConsider the following elements with their period number and valence electrons.
ElementsPeriod numberTotal valencee−P24Q26R37S33T36U34
According to the given informations, answer the following questions :
Choose incorrect statement :
View SolutionAn element of p-block in which last electron enters into s-orbital of valence shell insteatd of p-orbital is :
View SolutionThe general valence shell electronic configuration of p -block elements is
View SolutionWhich of the following relation is correct for boron element? Here P = period number of boron
N = number of valence electrons is boron
View SolutionHow does filling of valence electrons takes place in
p-block, d-block and f-block elements?
View SolutionExplain periodicity of valence or oxidation state of s and p block elements.
View SolutionGive the valence shell electronic configuration of P-block elements.
View SolutionWrite the valence shell electronic configuration of P-block elements.
View Solutions तथा p ब्लॉक के तत्वों की संयोजकता कैसे ज्ञात होती है ?
View SolutionIf P to T are second period p-block elements then which of the following graph show corrrect relation between valence electrons in P2→T2
View SolutionConsider the elements in the second period and p-block. The first ionization energy (of these elements)
View SolutionBohr's periodic table has seven horizontal rows called periods and eighteen vertical columns called groups. Alternatively, the elements of the Bohr's table are divided into four blocks namely S- and f-blocks. Group, period and block of given element can be predicted with the help of its electronic configuration.
(I) The principle quantum number of valence shell refers to period of elements
(II) The nature of subshell (S. p or d) having last electron refers the block to which the element belongs.
(III) The number of electrons present in outermost or penultimate shell predicts the group of element.
For s-block, the group number = No. of valence electron
For p-block, the group number =10+ numbers of valence electron
For d-block, the group number =2+(n−1)d electrons
Number of groups possible for s+p+d block elements in a period are
View Solution

