Drago suggested an emprical rule which is compatible with the energetics of hybridization. It will occupy a stereochemically inactive s-orbital, and the bonding will be through p-orbitals and bond angles will be nearly 90∘ if the electronegativity of the surrounding atoms is ≤2.5.
Q. In which of the following molecule central atom has higher % s-character in its bond pair:
AsH3
GeH3
P4
H2Se
The correct Answer is:B
Similar Questions

In all expected compounds each central atom only uses its s and p-orbitals in hybridization. The relationship between bond angle 'θ' and decimal fraction of s and p character present in the equivalent orbitals is given by :
cosθ=SS−1=P−1P, S=decimal fraction of s-character in the equivalent hybrid orbital and P=decimal fraction of p-character in the equivalent hybrid orbital.
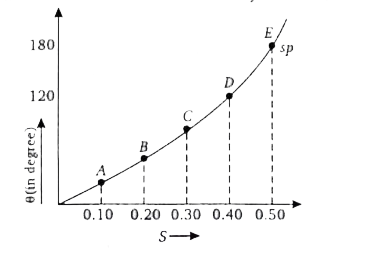 .
.
Q. The correct order of % p-character in bond pairs of central atoms in the following compounds is :
View SolutionDrago suggested an emprical rule which is compatible with the energetics of hybridization. It will occupy a stereochemically inactive s-orbital, and the bonding will be through p-orbitals and bond angles will be nearly 90^(@) if the electronegativity of the surrounding atoms is le2.5 . Q. In which of the following molecule central atom has higher % s-character in its bond pair:
View SolutionDrago suggested an emprical rule which is compatible with the energetics of hybridization. It will occupy a stereochemically inactive s-orbital, and the bonding will be through p-orbitals and bond angles will be nearly 90^(@) if the electronegativity of the surrounding atoms is le2.5 . Q. Correct order of bond angle is :
View SolutionTwo hybrid orbitals have a bond angle of 120^(@) . The percentage of p-character in the hybrid orbital is nearly:-
View SolutionAccording to Bent's rule, more electronegative atom prefers to stay in that hybrid orbital which has less s-character and lone pair prefers to stay in that hybrid orbital which has more s-character. Consider the molecules given below and answer the following question Which of the following bond length order is correct?
View SolutionIf the central atom is of third row or below this in the periodic table, then lone pair will occupy a stereochemically inactive s-orbital and bonding will be through almost pure p-orbitals and bond angles are nearly 90∘, if the substituent's electronegativity value is ≤2.5.
In which of the following option, covalent bond is having maximum s% character ?
View SolutionIf the central atom is of third row or below this in the periodic table, then lone pair will occupy a stereochemically inactive s-orbital and bonding will be through almost pure p-orbitals and bond angles are nearly 90∘, if the substituent's electronegativity value is ≤2.5.
Select incorrect statement regarding P4 molecule.
View SolutionIf the central atom is of third row or below this in the periodic table, then lone pair will occupy a stereochemically inactive s-orbital and bonding will be through almost pure p-orbitals and bond angles are nearly 90∘, if the substituent's electronegativity value is ≤2.5.
In which of the following option, covalent bond is having maximum s% character ?
View Solution

