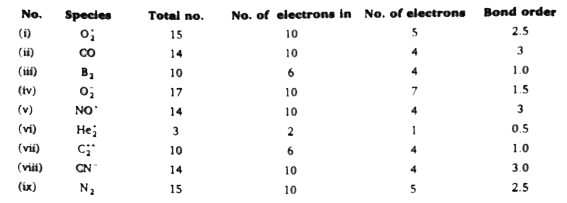
There are some species given below :-
(a)O+2(b)CO(c)B2(d)O+2(e)NO+(f)He+2(g)C+22(h)CN−(i)N−2
Total no. of species which have their fractional bond order.
The correct Answer is:4
→ molecular orbital configuration for upto 14 electrons
σ1s<*σ1s<σ2s<*σ2s<π2pxπ2py<σ2px<*π2px=*π2py<*simga2px
For more than 14 electrons σ1s<*σ1s<σ2s<*σ2s<π2px=π2py)<*π2px=*π2py<*π2px
Bond order =12 number of electron in [Bonding M.O.-Antibonding M.O.]
Similar Questions
There are some species given below. (i)O+2 (ii)CO (iii)B2 (iv)O−2 (v)NO+ (vi)He+2 (vii)C2+2 (vii)CN− (ix)N−2
total number of species which have fractional bond order.
View SolutionConsider the following species and find the total number of species which have fractional bond order : O+2 , CO,B2 , O−2, NO+ , He+2 , C2+2, CN− , N−2
View Solution
Knowledge Check
The species having bond order same as that of
(a)CO
(b) O2
(c)N2
(d) H2A(a) and(b)B(a) and(c)C(c) and(d)D(a), (b) and(d)
Identify the chemical species having identical bond order: O2+2,N2,O2,O2−2.
View SolutionHow many of the following species are paramagnetic?
N+2,O−2,B2,C2,CN−,NO+,CO,H−2,He+2
View SolutionThe number of molecules or ions having bond order 2.5 among O+2,CN,NO,N+2,CO+,NO+,O−2,CN−,N2, is
View SolutionThe number of paramagentic species among the following is
O2,CO,N2,C2,CsO2,BaO2,CO2
View SolutionNumber of acidic oxides among the following is:
(a) N2O (b) NO (c) N2O3 (d) N2O4 (e) N2O5 (f) P4O6
(g) P4O10 (h) SO3 (i) B2O3 (j) CO
View SolutionWhich of the following species have same bond order and same shape
(a) NΘ3 (b) O3 (c ) CO2 (d) NOΘ2.
View SolutionTotal number of species which have co-ordinate bond N2O5,XeF6,NH4,BF4,CO,C2H4
View Solution
