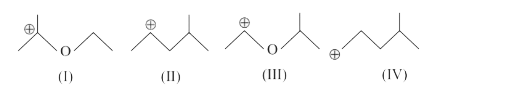
Choose incorrect stability order:
A
[Cu(NH3)4]2+<[Cu(en)2]2+<[Cu(trien)]2+
B
[Fe(H2O)6]3+<[Fe(NO2)6]3−<[Fe(NH3)6]3+
C
[Co(H2O)6]3+<[Rh(H2O)6]3+<[Ir(H2O)6]3+
D
[Cr(NH3)6]1+<[Cr(NH3)6]2+<[Cr(NH3)6]3+
Video Solution
Text Solution
The correct Answer is:B
[Fe(H2O)6]3+<[Fe(NO2)6]3−<[Fe(NH3)6]3+
NO−2 is stronger ligand than NH3.
|
Topper's Solved these Questions
- CO-ORDINATION COMPOUNDSBook:VK JAISWALChapter:CO-ORDINATION COMPOUNDSExercise:LEVEL 3 (PASSAGE TYPE)
Explore 97 Videos
- CO-ORDINATION COMPOUNDSBook:VK JAISWALChapter:CO-ORDINATION COMPOUNDSExercise:ASSERTION-REASON TYPE QUESTIONS
Explore 26 Videos
- CO-ORDINATION COMPOUNDSBook:VK JAISWALChapter:CO-ORDINATION COMPOUNDSExercise:SUBJECTIVE PROBLEMS
Explore 43 Videos
- CHEMICAL BONDING (BASIC)Book:VK JAISWALChapter:CHEMICAL BONDING (BASIC)Exercise:Level 3 (Passive 11)
Explore 6 Videos
- d-BLOCK ELEMENTSBook:VK JAISWALChapter:d-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 6 Videos
Similar Questions
Knowledge Check
The incorrect stability order of +3 and +1 states of 13th group elements (boron family) is:
AGa3+<In3+<Tl3+BTl+>Tl3+CGa+<In+<Tl+DGa3+>Ga+The correct stability order of cycloalkane is
Acyclopropane <cyclohexane<cyclopentaneBCyclohexane<cyclopentane<cyclopropaneCcyclopropane<cyclopentane<cyclohexaneDcyclopentane<cyclopropane<cyclohexaneThe correct stability order of following is :
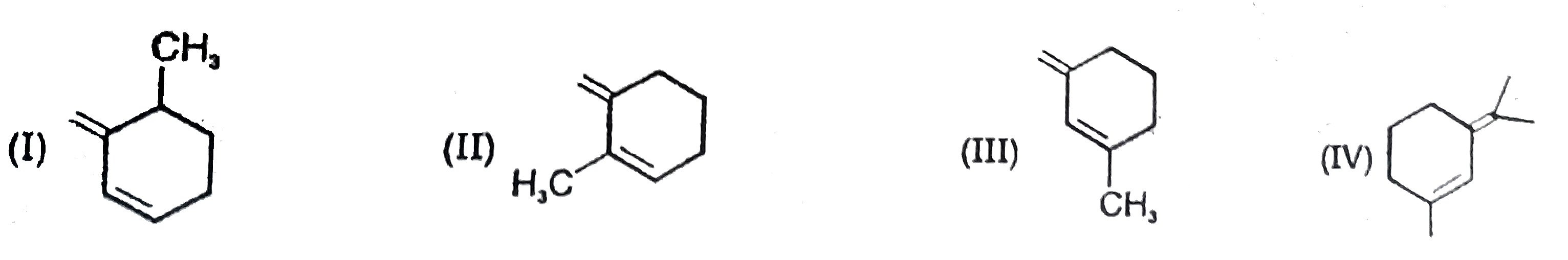 AIgtIIgtIIIgtIVBIIIgtIVgtIIgtICIIgtIVgtIIIgtIDIVgtIIIgtIIgtI
AIgtIIgtIIIgtIVBIIIgtIVgtIIgtICIIgtIVgtIIIgtIDIVgtIIIgtIIgtI
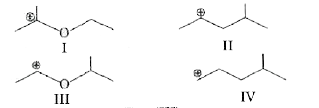
The correct stability order for the following species is
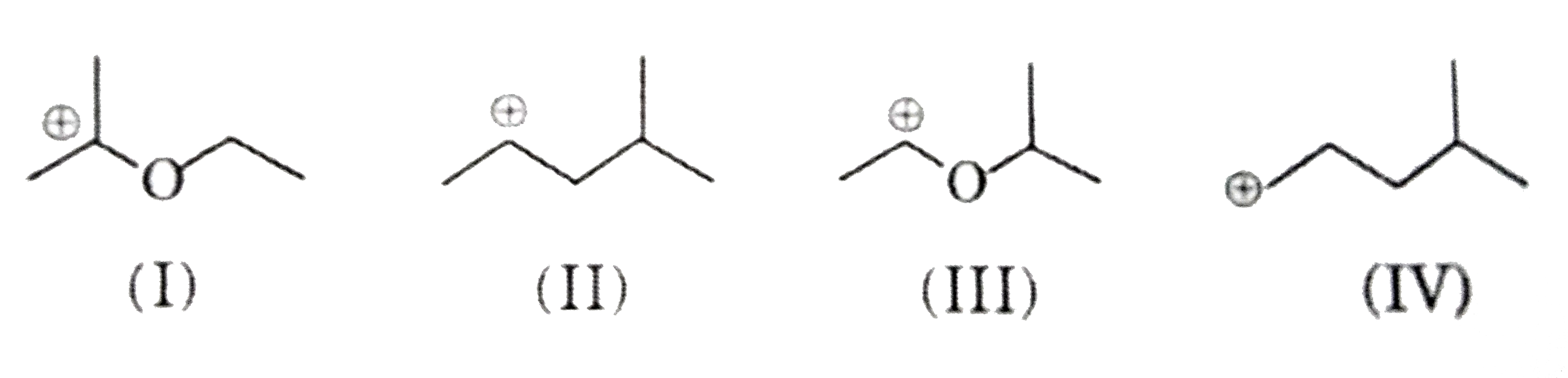
View SolutionThe correct stability order for the following species is
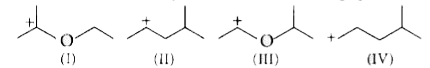
View SolutionThe correct stability order of the following species is :

View SolutionThe correct stability order for the following species is
View SolutionThe correct stability order of the following speicies is
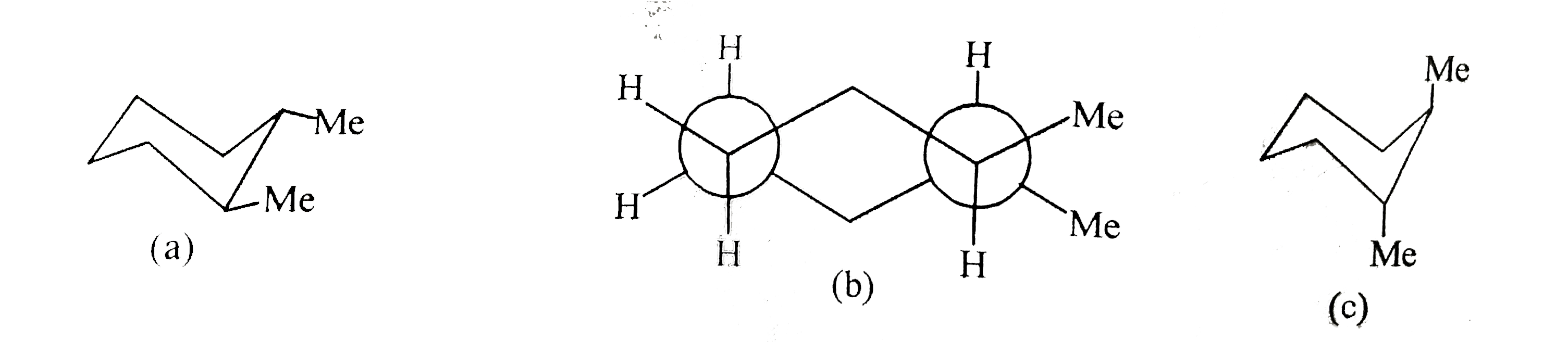
View Solution