Assertion : In the electrolytic reduction of Al2O3, cryolite is used.
Reason : Cryolite is an ore of aluminium.
If both assetion and reason are CORRECT, and reason is the CORRECT explanation of the assertion
If both assertion and reason are CORRECT, but reason is NOT the CORRECT explanation of the assertion
If assertion is CORRECT but reason is INCORRECT
If assertion is INCORRECT but reason is CORRECT
The correct Answer is:B
Step by step video, text & image solution for Assertion : In the electrolytic reduction of Al_(2)O_(3), cryolite is used. Reason : Cryolite is an ore of aluminium. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- METALLURGYBook:VK JAISWALChapter:METALLURGYExercise:MATCH THE COLUMN
Explore 16 Videos
- ENVIRONMENTAL CHEMISTRYBook:VK JAISWALChapter:ENVIRONMENTAL CHEMISTRYExercise:ASSERTION-REASON TYPE QUESTIONS
Explore 14 Videos
- p-BLOCK ELEMENTSBook:VK JAISWALChapter:p-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 36 Videos
Similar Questions
Column-I and Column-II contains four entries each. Entries of column-I are to be matched with some entries of column-II. Each entry of column-I may have the matching with one or more than one entries of column-II.

View SolutionAssertion (A) Green house effect was observed in houses used to grow plants and these are made of green glass.
Reason (R) Green house name has been given because glass houses are made of green glass.
View SolutionIn phosphorus acid, If X is number of non-bonding electron pairs. Y is number of σ-bonds and Z is number of π-bonds. Then calculate value of "YxZ-X".
View SolutionThe major role of flourspar, CaF_(2) which is added in small amount in the electrolytic reduction of Al_(2)O_(3) dissolved on fused cryolite in fused cryolite is
View SolutionAssertion: In Hall-Heroult process aluminium is extracted by the electrolytic reduction of alumina dissolved in molten cryolite or fluorspar.
Reason: The cryolite ore fluorspar lower the melting point of melt an dmake it more conducting
View SolutionExtraction of aluminium can be understood by :
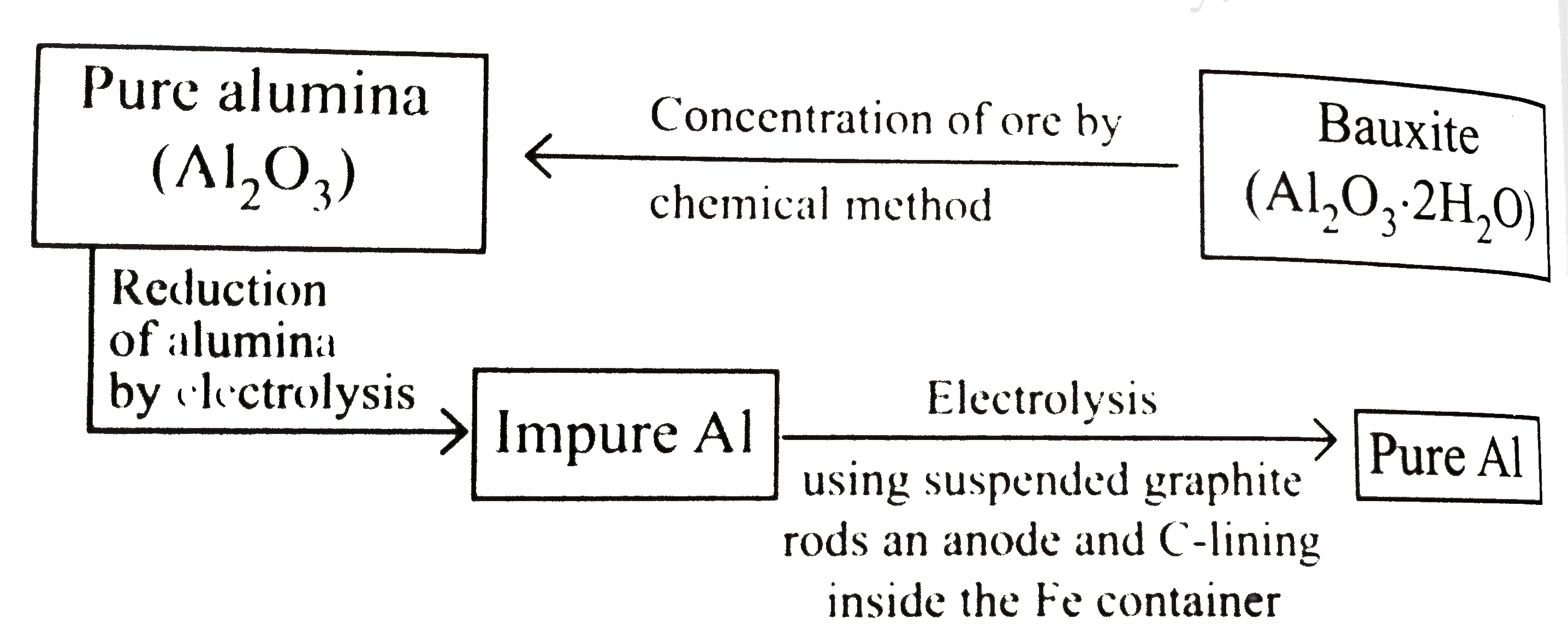
Electrolytric reduction of Al2O3 :
Electrolysis : Al2O3+Cryolite+CaF2
Cathode : Carbon inside the Fe container
Anode : Graphite rods.
The purpose of adding cryolite is.
View SolutionExtraction of aluminium can be understood by :
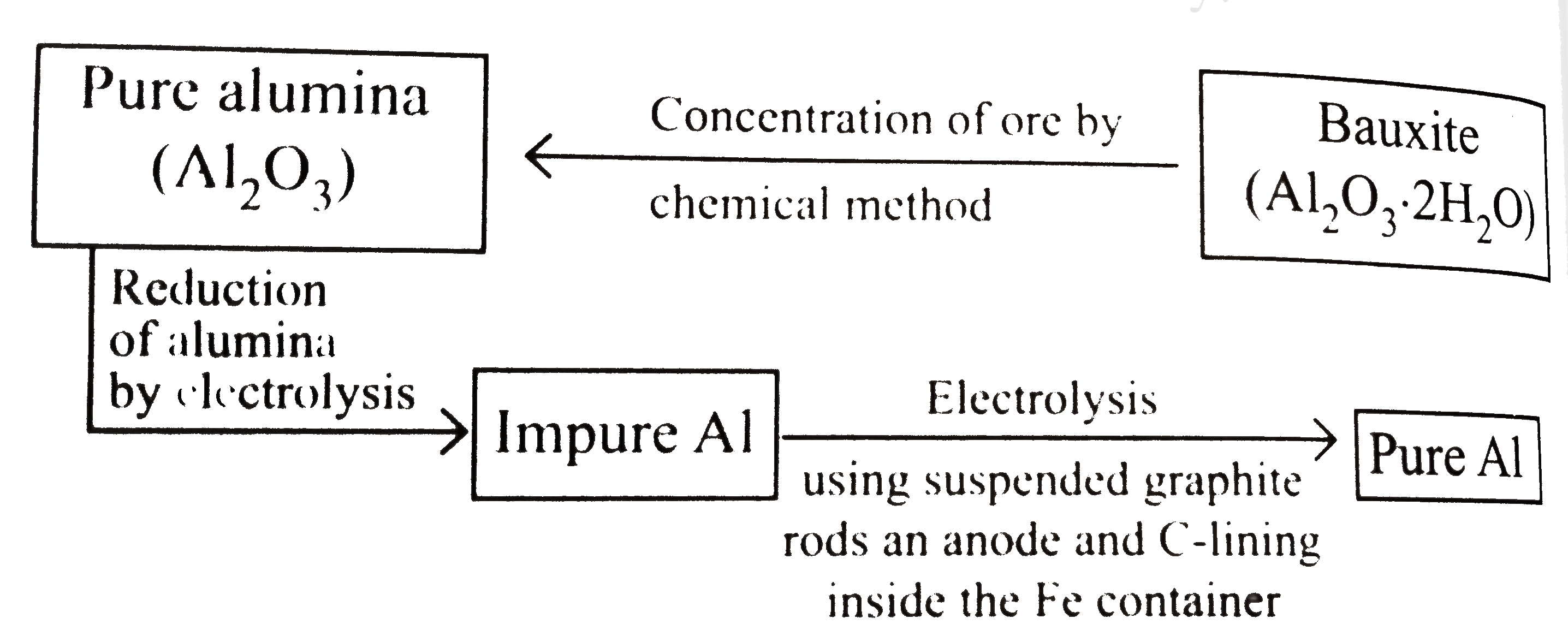
Electrolytric reduction of Al2O3 :
Electrolysis : Al2O3+Cryolite+CaF2
Cathode : Carbon inside the Fe container
Anode : Graphite rods.
Coke power is spread over the molten electroltyte to.
View SolutionAssertion : In the electrolytic reduction of Al2O3, cryolite is used.
Reason : Cryolite is an ore of aluminium.
View SolutionExtraction of aluminium can be understood by :

Electrolytric reduction of Al2O3 :
Electrolysis : Al2O3+Cryolite+CaF2
Cathode : Carbon inside the Fe container
Anode : Graphite rods.
The purpose of adding cryolite is.
View SolutionExtraction of aluminium can be understood by :

Electrolytric reduction of Al2O3 :
Electrolysis : Al2O3+Cryolite+CaF2
Cathode : Carbon inside the Fe container
Anode : Graphite rods.
The purpose of adding cryolite is.
View SolutionExtraction of aluminium can be understood by :

Electrolytric reduction of Al2O3 :
Electrolysis : Al2O3+Cryolite+CaF2
Cathode : Carbon inside the Fe container
Anode : Graphite rods.
Coke power is spread over the molten electroltyte to.
View SolutionAssertion : In the electrolytic reduction of Al2O3, cryolite is used.
Reason : Cryolite is an ore of aluminium.
View SolutionExtraction of aluminium can be understood by :

Electrolytric reduction of Al2O3 :
Electrolysis : Al2O3+Cryolite+CaF2
Cathode : Carbon inside the Fe container
Anode : Graphite rods.
The purpose of adding cryolite is.
View SolutionStatement-1:In the electrolytic reduction of Al2O3, cryolite lowes the melting point of the mixture and bring conductivity:
Statement-2: Cryolite is an ore of aluminium.
View SolutionThe major role of fluorspar, which is added in small quantities in the electrolytic reduction of Al2O3 dissolved in fushed cryolite is
View Solution
