On treatment with cold water, an element (A) reacts readily liberating a colourless, odourless gas (B) and a solution (C). Lithium is reacted with (B) yeildng a solid product (D) which effervesce with water to give a strongely basic solution (E). When CO2 gas is bubbled through solution (C), a white ppt. (F) is formed but this redissolved forming solution (G) when more CO2 is passed. Precipitate (F) effervesced when moistened with conc. HCl and give deep red colouration to Bunsen burner flame. (F) on heating with excess of carbon at 2000∘C give (H).
Answer the following question on the basis of above passage.
Q. Solution (G) contains a salt which
(i) causes permanent hardness of water
(ii)can not be obtained in solid state
(iii)causes temporary hardness of water
(iv)can be obtained in solid state
Select the correct statements :
(i) and (ii)
(i) and (iv)
(ii) and (iii)
(ii) and (iv)
The correct Answer is:C
It is CaHCO3, which can not be obained in solid state and causes temporary hardness
Topper's Solved these Questions
- s-BLOCK ELEMENTSBook:VK JAISWALChapter:s-BLOCK ELEMENTSExercise:ONE OR MORE ANSWER IS/ARE CORRECT
Explore 69 Videos
- s-BLOCK ELEMENTSBook:VK JAISWALChapter:s-BLOCK ELEMENTSExercise:ASSERTION-REASON TYPE QUESTIONS
Explore 34 Videos
- s-BLOCK ELEMENTSBook:VK JAISWALChapter:s-BLOCK ELEMENTSExercise:SUBJECTIVE PROBLEMS
Explore 2 Videos
- QUALITATIVE INORGANIC ANALYSISBook:VK JAISWALChapter:QUALITATIVE INORGANIC ANALYSISExercise:SUBJECTIVE PROBLEMS
Explore 5 Videos
- TYPES OF REACTIONSBook:VK JAISWALChapter:TYPES OF REACTIONSExercise:SUBJECTIVE PROBLEMS
Explore 42 Videos
Similar Questions
The correct statement(s) is /are
View SolutionStatement-I : Li2SO4 do not form double salt like alum.
Statement-II : Li reacts with NH3 gas to form LiNH2
View Solution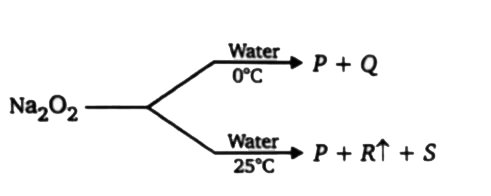
Calculate sum of bond order between same bonded atoms in Q and R compounds.
View SolutionConsider the following reaction
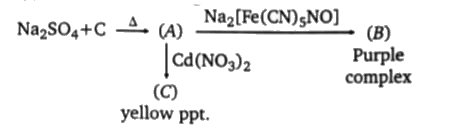
Then calculate value of ∣∣X2−Y2∣∣ (where X and Y are total number of electrons present t_(2g) and e_(g) orbitals respectively in d-block metal ion of compound B).
View SolutionFind total number of reagnets which cann produce I2 from KI solution.
Conc. H2SO4, Hg(NO3)2solution, CuSO4 solution, Conc.H3PO4,
K2Cr2O7/H+, Cl2 Water, Pb(CH3COO)2 solution, Ca(OCl)Cl/H+, NaNO2+dil.HCl
View SolutionOn treatment with cold water, an element (A) reacted quietly liberating a colourless, odourless gas (B) and a basic solution (C). Lithium reacted with (B) yielding a solid product (D) which effervesced with water to give a stongly basic solution (E) and gas (F). When CO2 was bubbled through solution (C), initially a white ppt. (G) was formed, but this redissolved forming solution (H) when more CO2 was passed, precipitate (G) effervesced when moistened with conc HCl and gave a brick red colouration to the bunsen flame. When (G) was heated with carbon at 1000∘C, a caustic white compound (I) was formed, which when heated with N20 at 1000∘C gave a solid (J) of some commercial importance. Identify (A) to (J) and explain the reactions.
View SolutionA colourless (A) when place into water a heavy white turbidly of (B) solid (A) gives a close solution in conesolution in cone HCI when HCI solution is added to clear solution water ,(B) forms again (B) dissolves in dilute HCI. When H2S is passed through a sespension of (A) or (B), a black precipitate (C ) forms , (C ) is insolves in yellow ammonium sulphide (NH4)2S , cone H2SO4 added to solid (A) liberates gas (D) gas (D) is water soluble and gives white precipitate with mercuric salts (E ) and not mercuric salt .The black precipitate (C ) dissolves in HNO3,(1,1) to give a solution to which H2SO4 is added followed by addition of NH4OH when a white precipitate (F) is formed (E ) gives a black ppt , (G) with solution of sodium stannite.
When compound (E ) reacts with NH4OH , then product is a
View SolutionA colourless (A) when place into water a heavy white turbidly of (B) solid (A) gives a close solution in conesolution in cone HCI when HCI solution is added to clear solution water ,(B) forms again (B) dissolves in dilute HCI . When H_(2)S is passed through a sespension of (A) or (B), a black precipitate (C ) forms , (C ) is insolves in yellow ammonium sulphide (NH_(4))_(2)S , cone H_(2)SO_(4) added to solid (A) liberates gas (D) gas (D) is water soluble and gives white precipitate with mercuric salts (E ) and not mercuric salt .The black precipitate (C ) dissolves in HNO_(3), (1,1) to give a solution to which H_(2)SO_(4) is added followed by addition of NH_(4)OH when a white precipitate (F) is formed (E ) gives a black ppt , (G) with solution of sodium stannite. Compound (C ) is also formed by the following reaction
View SolutionOn treatment with cold wate, element (A) reacted quietly, librating colourless gas (B) and solution ( C). Lithium reacted with gas (B) and giving a solid product (D) which gave effervesence with water to give basic solution (F). When CO2 was passed thorough solution ( C), an initial whit ppt (E) was formed but this redissolved forming solution (G) when more CO2 was passed. PPT (E) effervessced when moistened to Bunsen flame. (E ) on heating wiht excess of carbon at 2000∘C gave (H):
Metal A may be:
View SolutionOn treatment with cold wate, element (A) reacted quietly, librating colourless gas (B) and solution ( C). Lithium reacted with gas (B) and giving a solid product (D) which gave effervesence with water to give basic solution (F). When CO_(2) was passed thorough solution ( C), an initial whit ppt (E) was formed but this redissolved forming solution (G) when more CO_(2) was passed. PPT (E) effervessced when moistened to Bunsen flame. (E ) on heating wiht excess of carbon at 2000^(@)C gave (H): Solution (G) contains a salt. Consider following statements: I) It causes permanent hardness of water. II) It can't be obtained in solid state. III) It causes temporary hardness to water. IV) It can be obtained in solid state. Correct statements are:
View SolutionOn treatment with cold wate, element (A) reacted quietly, librating colourless gas (B) and solution ( C). Lithium reacted with gas (B) and giving a solid product (D) which gave effervesence with water to give basic solution (F). When CO_(2) was passed thorough solution ( C), an initial whit ppt (E) was formed but this redissolved forming solution (G) when more CO_(2) was passed. PPT (E) effervessced when moistened to Bunsen flame. (E ) on heating wiht excess of carbon at 2000^(@)C gave (H): Incorrect statement among the following is
View SolutionA colourless solide (A) on hydroglysis produces a white ppt. (B) of heavy metal Solid (A) gives a clear solution in concentration HCl, however, when added to large amount of water, it again gives precipitate of (B), when H_(2)S in passed through a suspension of (A) and (B), a brown black ppt of (C ) is obtained. Compound (A) liberates a gas (D) on treating with H_(2)SO_(4) . The gas (D) is water soluble and gives white ppt. (E) with solution of mercurous salt but not with mercuric salt. The ppt white ppt (B) is of
View SolutionA colourless solide (A) on hydroglysis produces a white ppt. (B) of heavy metal Solid (A) gives a clear solution in concentration HCl, however, when added to large amount of water, it again gives precipitate of (B), when H_(2)S in passed through a suspension of (A) and (B), a brown black ppt of (C ) is obtained. Compound (A) liberates a gas (D) on treating with H_(2)SO_(4) . The gas (D) is water soluble and gives white ppt. (E) with solution of mercurous salt but not with mercuric salt. The gas (D) might be
View SolutionA colourless solide (A) on hydroglysis produces a white ppt. (B) of heavy metal Solid (A) gives a clear solution in concentration HCl, however, when added to large amount of water, it again gives precipitate of (B), when H_(2)S in passed through a suspension of (A) and (B), a brown black ppt of (C ) is obtained. Compound (A) liberates a gas (D) on treating with H_(2)SO_(4) . The gas (D) is water soluble and gives white ppt. (E) with solution of mercurous salt but not with mercuric salt. White ppt (E) is of
View SolutionOn treatment with cold water, an element (A) reacts readily liberating a colourless, odourless gas (B) and a solution (C). Lithium is reacted with (B) yeildng a solid product (D) which effervesce with water to give a strongely basic solution (E). When CO_(2) gas is bubbled through solution (C), a white ppt. (F) is formed but this redissolved forming solution (G) when more CO_(2) is passed. Precipitate (F) effervesced when moistened with conc. HCl and give deep red colouration to Bunsen burner flame. (F) on heating with excess of carbon at 2000^(@) C give (H). Answer the following question on the basis of above passage. Q. Solid (H) on hydrolysis gives a gas which on passing through ammoniated AgNO_(3) solution yields:
View Solution
