Material: A tray with thick layer of wax, solid spheres of iron, lead and copper of equal mass, burner or spirit lamp, large beaker.
Procedure:
i. Take three spheres of iron, copper and lead of equal mass.
Depth of each sphere in wax slab is different.
Similar Questions
Material: A tray with thick layer of wax, solid spheres of iron, lead and copper of equal mass, burner or spirit lamp, large beaker.
Procedure:
ii. Put all the three spheres in both water m the beaker for same time.
View SolutionMaterial: A tray with thick layer of wax, solid spheres of iron, lead and copper of equal mass, burner or spirit lamp, large beaker.
Procedure:
ii. Put all the three spheres in both water m the beaker for same time.
View Solution
Knowledge Check
The M.I. of two spheres of equal masses about their diameters are equal. If one of them is solid and other is hollow, the ratio of their radius is
A√3:√5B3:5C√5:√3D5:3The moment of inertia of a dumb bell consisting of two identical uniform solid spheres of mass m and radius R each, joined by a thin metallic rod of equal mass m (separation between the centres of the spheres is 6R) is I about the axis AB. Its moment of inertia, about an axis making an angle of 45∘ with AB, is
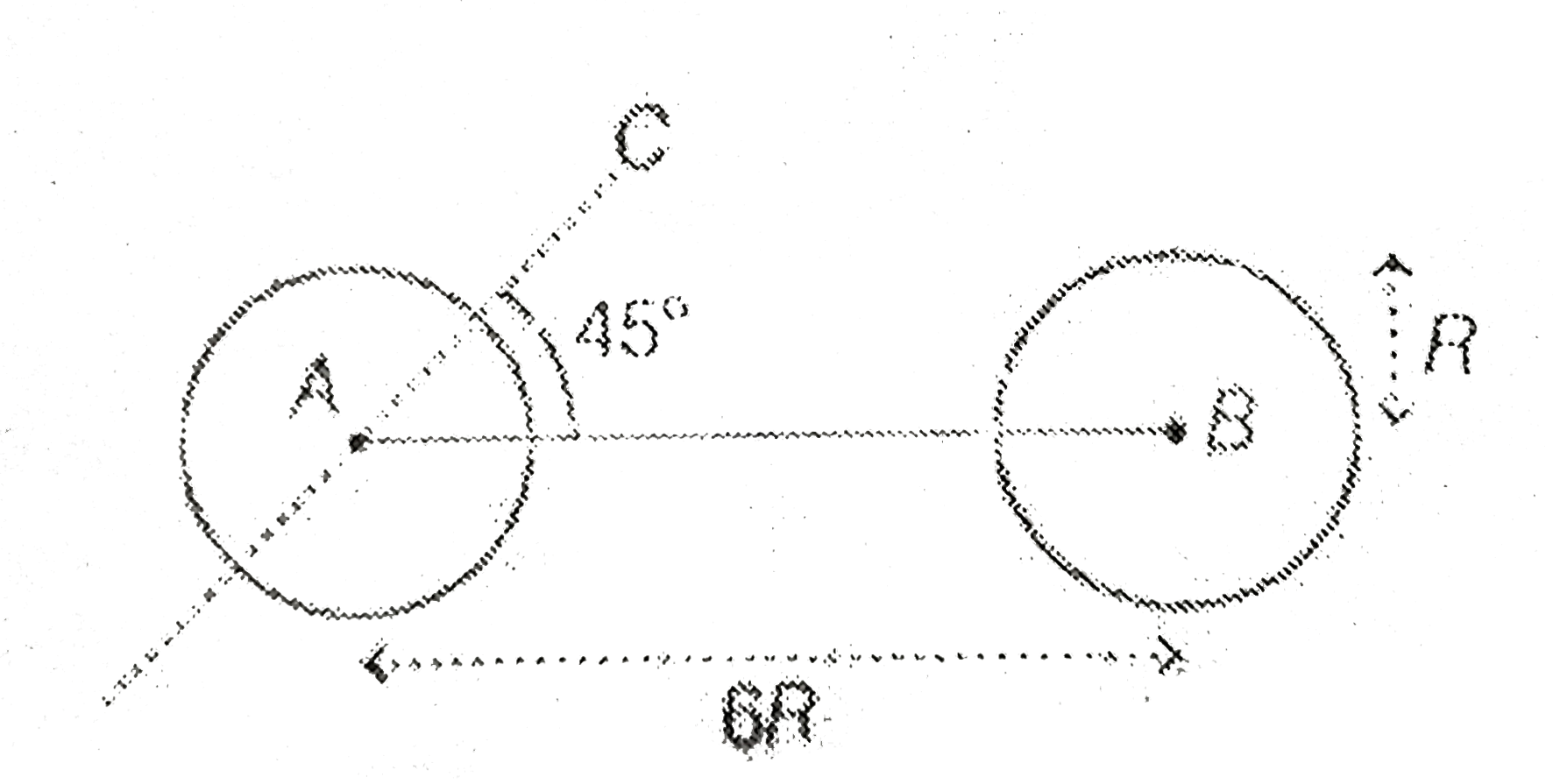 A372lB945lC31lD772l
A372lB945lC31lD772l
Study the following procedure and answer the question below :
1. Take 3 spheres of iron , copper and lead of equal mass.
2. Put all the 3 spheres in boiling water in a beaker for some time.
3. Take 3 spheres out of the water. Put them immediately on a thick slab of wax.
4. Note the dept that each sphere goes into the wax.
(i) Which property of a substance can be studied with this procedure ? ltbr> (ii) Describe that property in minimum words.
(iii) Explain the rule of heat exchange with this property.
View SolutionApparatus: Copper wire, iron nail, beaker or big test tube, etc.
Chemicals: Aqueous solutions of ferrous sulphate and copper sulphate.
Procedure: i. Take a clean copper wire and a clean iron nail.
ii. Dip the copper wire in ferrous sulphate solution and the iron nail in copper sulphate solution.
iii . Keep on observing continually at a fixed interval of time.
What is the type of the reaction?
View SolutionApparatus: Copper wire, iron nail, beaker or big test tube, etc.
Chemicals: Aqueous solutions of ferrous sulphate and copper sulphate.
Procedure: i. Take a clean copper wire and a clean iron nail.
ii. Dip the copper wire in ferrous sulphate solution and the iron nail in copper sulphate solution.
iii . Keep on observing continually at a fixed interval of time.
In which test tube a reaction has taken place?
View SolutionApparatus: Copper wire, iron nail, beaker or big test tube, etc.
Chemicals: Aqueous solutions of ferrous sulphate and copper sulphate.
Procedure: i. Take a clean copper wire and a clean iron nail.
ii. Dip the copper wire in ferrous sulphate solution and the iron nail in copper sulphate solution.
iii . Keep on observing continually at a fixed interval of time.
How did you recognize that a reaction has taken place?
View SolutionWe have three solid bodies of same material A rightarrow a solid cube of edge length 'r' Brightarrow a solid sphere of radius 'r' and C rightarrow a solid hemisphere of radius 'r' In coloumnI certain situation related to these bodies are given Match the appropriate outcome indicated in column-II
Column-IColumn-II(A)All 3 bodies are heated to some temperature of 350k and kept in a room at300k Then rate of fall of temperature with time(P)for C is highest(Q)for B is highest(B)All 3 bodies are kept on level groundC is kept with base on ground height of centre of mass from ground(R)for C is lowest(C)All 3 bodies are rotated about an axis passing through their repective centre of mass the axisfor cube and hemisphere is perpendicular to the fase and base respectively Moment of inertia(S) for one of the body is half of another body
View SolutionApparatus: Pairs of tongs or spatula, knife, burner, etc.
Chemicals: Samples of aluminium, copper, iron, lead, magnesium, zinc and sodium.
Procedure: Hold the sample of each of the above metals at the top of the flame of a burner with the help of a pair of tongs, or a spatula
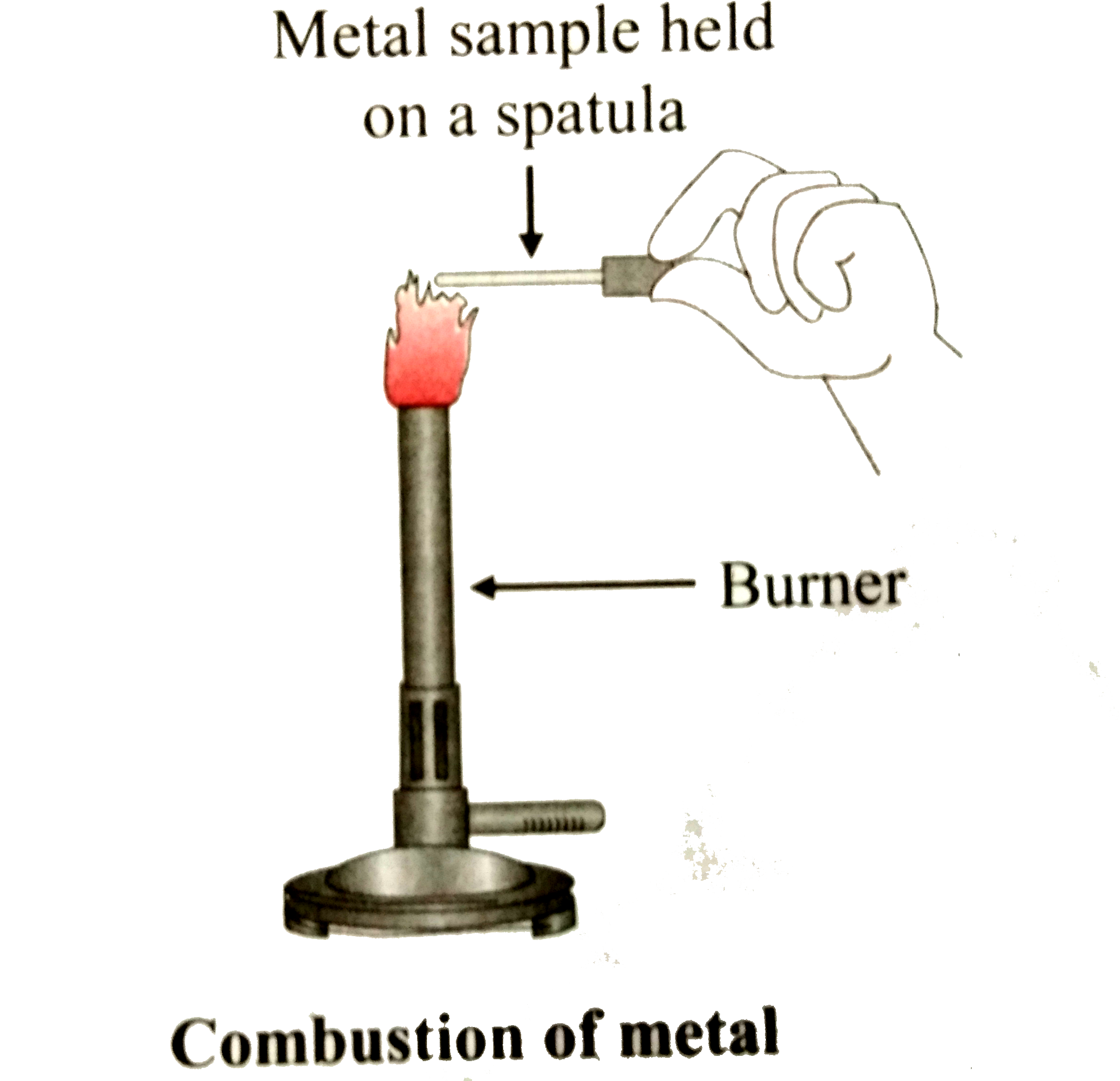
What is the colour of the flame while the metal is burning on the flame?
View Solution

