In the Williamson synthesis of ethers given by the general equation.
R−X+R'ONa→R−O−R' the order of reaction of RX is __________.
CH3>1∘>2∘>3∘
CH3<1∘<2∘<3∘
CH3<1∘<2∘>3∘
CH3>1∘<2∘<3∘
The correct Answer is:A
To determine the order of reaction of alkyl halides (RX) in the Williamson synthesis of ethers, we need to analyze the mechanism involved in this reaction.
1. Understanding the Reaction:
The Williamson synthesis involves the reaction of an alkyl halide (RX) with sodium alkoxide (R'ONa) to form an ether (R-O-R'). The general equation is:
R−X+R′ONa→R−O−R′+NaX
2. Identifying the Mechanism:
The reaction proceeds via an SN2 mechanism. In this mechanism, the nucleophile (alkoxide ion, R'O-) attacks the electrophilic carbon atom of the alkyl halide (RX) from the opposite side of the leaving group (X).
3. Analyzing Steric Hindrance:
The rate of the SN2 reaction is significantly influenced by steric hindrance. The more sterically hindered the carbon atom attached to the halogen (X), the slower the reaction will be.
4. Order of Reactivity:
- Methyl Halide (CH3X): No steric hindrance, fastest reaction.
- Primary Halide (RCH2X): Low steric hindrance, reacts quickly.
- Secondary Halide (R2CHX): Moderate steric hindrance, slower reaction.
- Tertiary Halide (R3CX): High steric hindrance, very slow reaction or may not react via SN2 at all.
5. Conclusion:
Based on the analysis, the order of reactivity of alkyl halides in the Williamson synthesis is:
Methyl > Primary > Secondary > Tertiary
Final Answer:
The order of reaction of RX in the Williamson synthesis of ethers is Methyl > Primary > Secondary > Tertiary.
---
The Williamson synthesis involves the reaction of an alkyl halide (RX) with sodium alkoxide (R'ONa) to form an ether (R-O-R'). The general equation is:
R−X+R′ONa→R−O−R′+NaX
The reaction proceeds via an SN2 mechanism. In this mechanism, the nucleophile (alkoxide ion, R'O-) attacks the electrophilic carbon atom of the alkyl halide (RX) from the opposite side of the leaving group (X).
The rate of the SN2 reaction is significantly influenced by steric hindrance. The more sterically hindered the carbon atom attached to the halogen (X), the slower the reaction will be.
- Methyl Halide (CH3X): No steric hindrance, fastest reaction.
- Primary Halide (RCH2X): Low steric hindrance, reacts quickly.
- Secondary Halide (R2CHX): Moderate steric hindrance, slower reaction.
- Tertiary Halide (R3CX): High steric hindrance, very slow reaction or may not react via SN2 at all.
Based on the analysis, the order of reactivity of alkyl halides in the Williamson synthesis is:
Methyl > Primary > Secondary > Tertiary
The order of reaction of RX in the Williamson synthesis of ethers is Methyl > Primary > Secondary > Tertiary.
Similar Questions
Knowledge Check
The reaction,
RX−R'−ONa→R'−O−R+NaX is calledAWurtz reactionBWilliamson's synthesisCKolbe's reactionDHofmann bromamide reaction.R.E.R is specialised for synthesis of
ALocal proteinsBLocal proenzymesCProteins and proenzymes for transportDHormonesThe R in the given reaction sequence is −
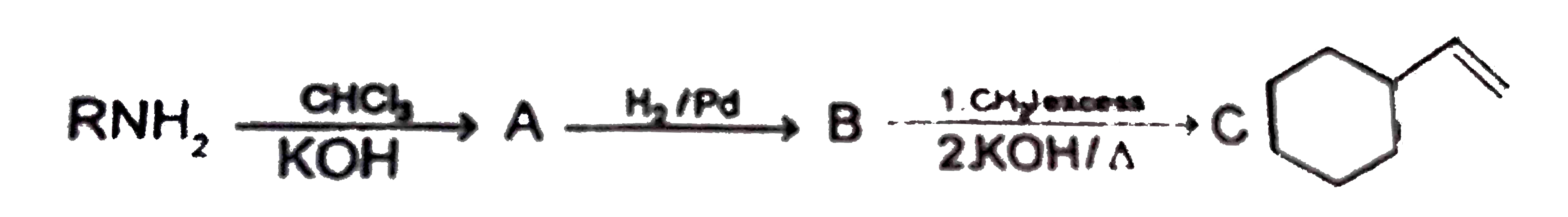 A
A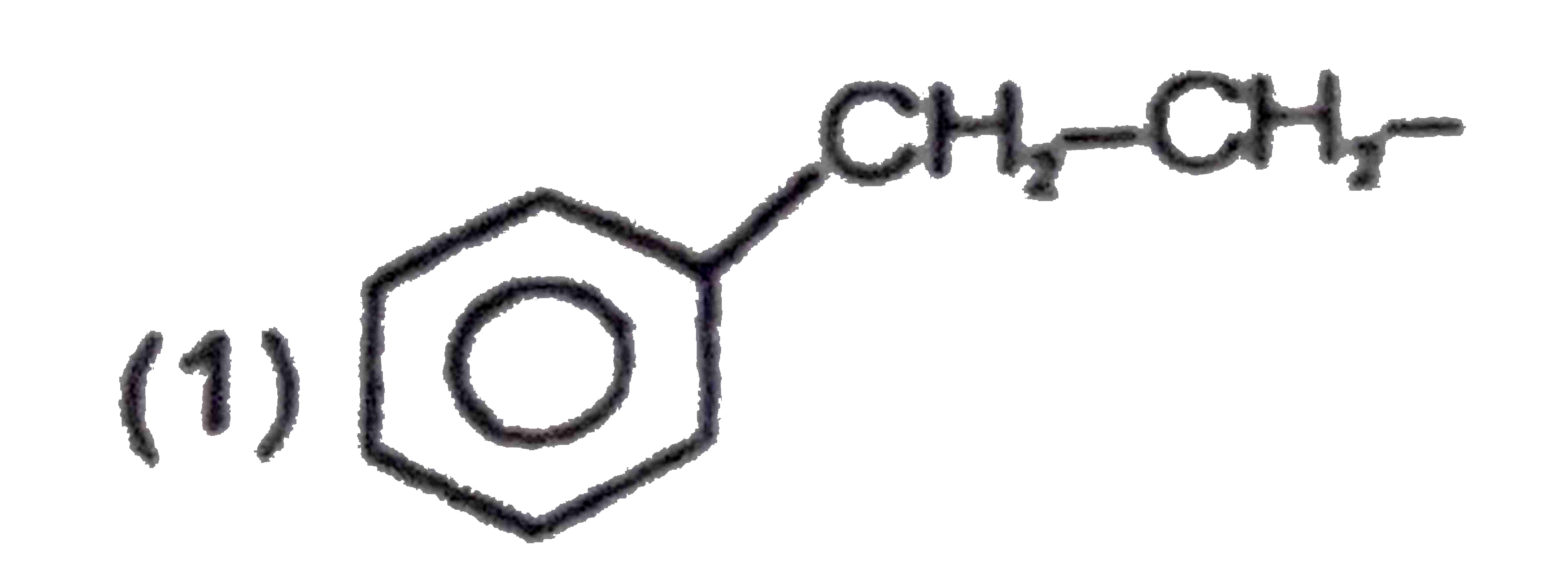 B
B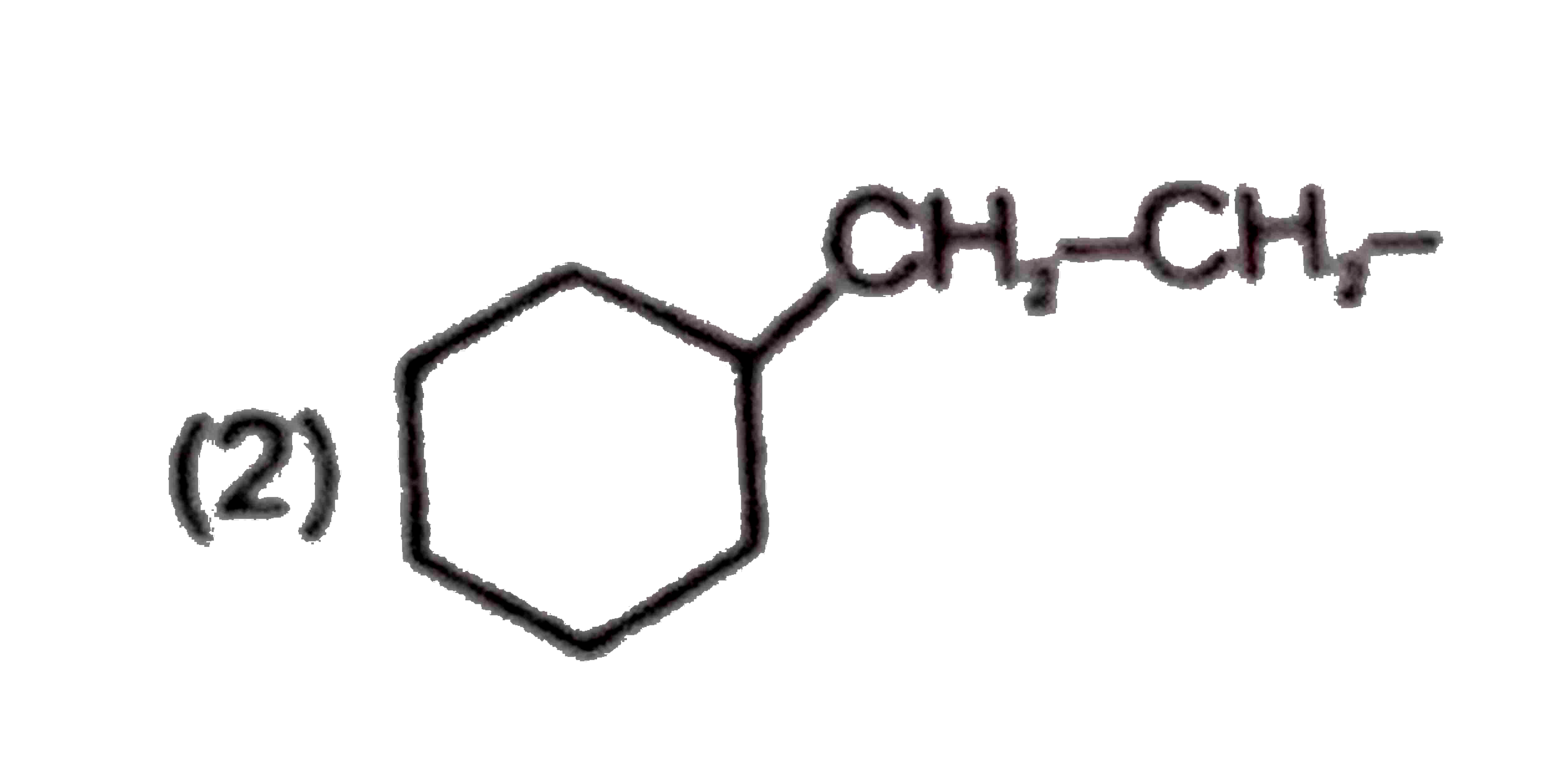 C
C D
D
In the preparation of ether, one of the reactant is R-X another is
View SolutionFor the reaction R−Br→R−O−N=O
the suitable reagent is
View SolutionReaction of R '- OH with R' - Mg X produces :
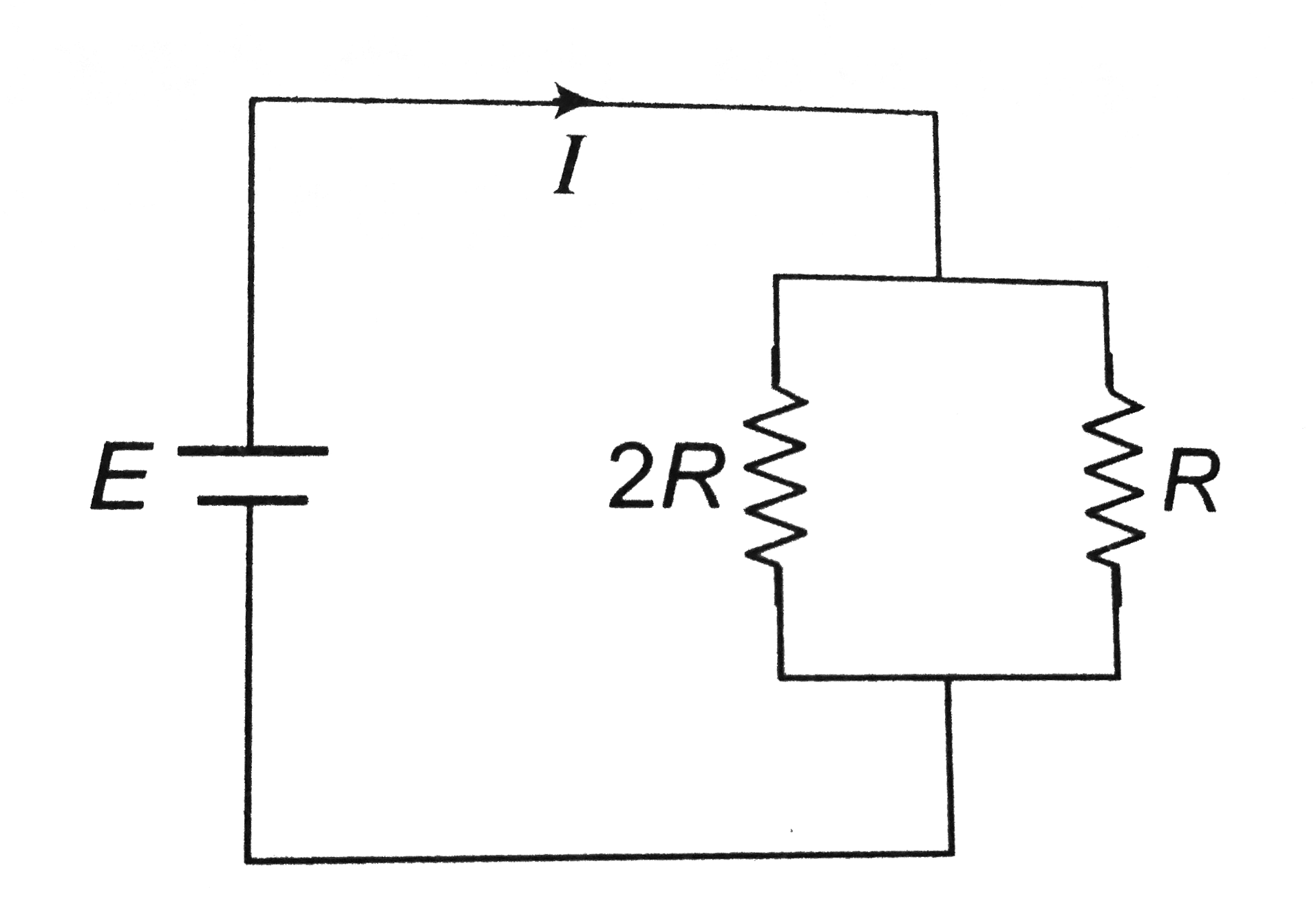
View SolutionWhat is the ratio of heat generated in R and 2R?
View SolutionSelect incorrect statement about following reaction of ether synthesis :
R-X + R' O Na → ROR' + NaX
View Solution

