The molecular formula of compound is C4H10O, is represents three isomers, then the type of isomerism is ___________.
functional isomerism
metamerism
chain isomerism
all of these
The correct Answer is:B
Step by step video, text & image solution for The molecular formula of compound is C_4H_(10)O, is represents three isomers, then the type of isomerism is ___________. by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- ALCOHOLS, PHENOLS AND ETHERS BOOK - TARGET PUBLICATIONCHAPTER - ALCOHOLS, PHENOLS AND ETHERS EXERCISE - EVALUATION TEST
21 Videos
- ALCOHOLS, PHENOLS AND ETHERS BOOK - TARGET PUBLICATIONCHAPTER - ALCOHOLS, PHENOLS AND ETHERS EXERCISE - CRITICAL THINKING
107 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDSBOOK - TARGET PUBLICATIONCHAPTER - ALDEHYDES, KETONES AND CARBOXYLIC ACIDSEXERCISE - EVALUATION TEST
19 Videos
Similar Questions
(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N.
(ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines ?
View Solution(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N.
ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
View Solution
Knowledge Check
Molecular formula C4H10O represent
ATwo primary alcoholBOne secondary alcoholCOne tertiary alcoholDAll of theseMolecular formula C2H4O2 represents
AacidsBestersCaldehydesDboth 'a' and 'b'Molecular formula C4H10O has ........ isomeric ethers ,
A4B3C7D5
(i) Write structures of different isomeric amines corresponding to the molecular formula, C4H11N.
ii) Write IUPAC names of all the isomers.
(iii) What type of isomerism is exhibited by different pairs of amines?
View SolutionCompounds with same molecular formula but differing in their structures are said to be structural isomers. What type of structural isomerism is shown by
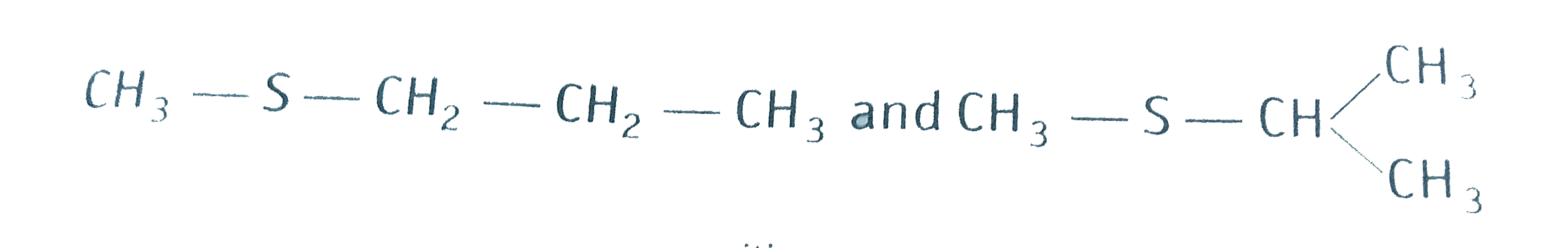
View SolutionA compound with molecular formula C4H10O, can show metamerism, functional isomerism and positional isomerism. Justify the statement.
View SolutionMolecular formula of a hydrocarbon is C5H10.What type of isomerisms are exhibited by it ? Give the structural formula of the isomes .
View SolutionThe number of isomers obtained from the formula C3H8O is
View Solution

