A reagent that is required to convert methyl cyanide into acetaldehyde is ________.
ethyl magnesium chloride
methyl magnesium chloride
acetyl chloride
SnCl2/HCl
The correct Answer is:D
To convert methyl cyanide (also known as acetonitrile) into acetaldehyde, we need to follow a specific chemical reaction. Here’s the step-by-step solution:
Step 1: Identify the Reactant and Product
- Reactant: Methyl cyanide (CH₃CN)
- Product: Acetaldehyde (CH₃CHO)
Step 2: Understand the Required Reaction
To convert methyl cyanide to acetaldehyde, we can use a reagent that facilitates the transformation of the nitrile group (-CN) into an aldehyde group (-CHO).
Step 3: Select the Appropriate Reagent
The reagent that can achieve this transformation is SnCl₂ (tin(II) chloride) in the presence of HCl. This combination is known for its ability to convert nitriles into aldehydes.
Step 4: Mechanism of the Reaction
1. Formation of an Imine: The SnCl₂ in HCl first reacts with the nitrile to form an imine intermediate.
2. Hydrolysis: Upon hydrolysis, the imine is converted into the corresponding aldehyde, which in this case is acetaldehyde.
Step 5: Conclusion
Thus, the reagent required to convert methyl cyanide into acetaldehyde is SnCl₂ in the presence of HCl.
Final Answer
The reagent that is required to convert methyl cyanide into acetaldehyde is SnCl₂ in the presence of HCl.
---
- Reactant: Methyl cyanide (CH₃CN)
- Product: Acetaldehyde (CH₃CHO)
To convert methyl cyanide to acetaldehyde, we can use a reagent that facilitates the transformation of the nitrile group (-CN) into an aldehyde group (-CHO).
The reagent that can achieve this transformation is SnCl₂ (tin(II) chloride) in the presence of HCl. This combination is known for its ability to convert nitriles into aldehydes.
1. Formation of an Imine: The SnCl₂ in HCl first reacts with the nitrile to form an imine intermediate.
2. Hydrolysis: Upon hydrolysis, the imine is converted into the corresponding aldehyde, which in this case is acetaldehyde.
Thus, the reagent required to convert methyl cyanide into acetaldehyde is SnCl₂ in the presence of HCl.
The reagent that is required to convert methyl cyanide into acetaldehyde is SnCl₂ in the presence of HCl.
Similar Questions
Knowledge Check
Collin's reagent is used to convert
A B−CH2OH→CHOC−CHO→−CHOD−CHO→−CH2OH
B−CH2OH→CHOC−CHO→−CHOD−CHO→−CH2OHThe reagents used to convert
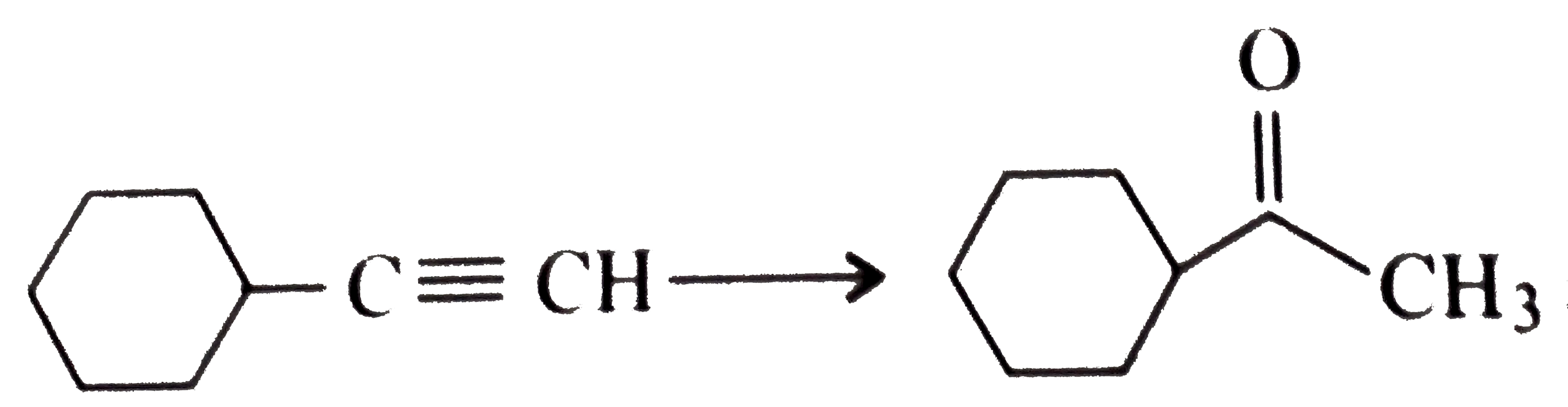 Ai. O3/Red. Ii. AlCl3 iii. MeCOOHBi. H2SO4+HgSO4 ii. H2O, heatCi. O3/Zn−AcOH ii. H2SO4+HgSO4 iii. H2O, heatDi. CH3COOH ii. H2O2+ΘOH/H2O
Ai. O3/Red. Ii. AlCl3 iii. MeCOOHBi. H2SO4+HgSO4 ii. H2O, heatCi. O3/Zn−AcOH ii. H2SO4+HgSO4 iii. H2O, heatDi. CH3COOH ii. H2O2+ΘOH/H2OMedius reduction converts an alkyl cyanide to
Aa primary amineBan aldehydeCa ketoneDan oxime.
How will you convert ethyl cyanide to ethanoic acid ?
View SolutionThe reagent required to convert 2-chloro-2 methyl butane into 2-methyl but 2-ene is
View SolutionLiAlH4 / ether reduces methyl cyanide into
View SolutionThe reagent that gives an orange coloured precipitate with acetaldehyde is
View SolutionThe reagents needed to convert is/are
View Solution
