Compound A i.e. an aromatic hydrocarbon of molecular mass 78gmol−1 is treated with compound 'B' with molecular mass 78.5gmol−1 in the presence of anhydrous AlCl3 to give compound 'C' . Compound 'C' gives positive test but shows negative results with Fehling solution, Tollen's reagent and Schiff reagent . Identify compound A and B.




The correct Answer is:B
Topper's Solved these Questions
Similar Questions
Empirical formula of a compound X (Molar mass =78mol−1 ) is CH. Write its molecular formula.
View SolutionA nitraite on acid hydrolysis gives compound A, which reacts with thionyl chloride to give compound B. Benzene reacts with compound B in presence of anhydrous AlCl3 to give compound C. The compound B reacts with ethyl alcohol to give ester D. Identify the compounds A,B C and D and write the equations.
View Solution
Knowledge Check
The compounds 'A' undergoes Koble's reaction in the presence of CO2 and NaOH to give compound 'B' . Compound 'B' when treated with acetic anhydride in acidic medium , gives acetylsalicylic acid i.e. aspirin. Identify the compound 'A' and 'B'.
ASalicyladehyde, Salicylic acidBPhenol, salicylic acidCphenol, salicyladehydeDPhenol , BenzaldehydeAn optically active compound C6H12O gives positive test with 2, 4-dinitrophenyl hydrazine , but negative with Tollen's reagent , what is the structure of the compound?
ACH3−O∣∣C−CH2−CH2−CH2−CH3BH−O∣∣C−CH∣CH3−CH2−CH2−CH3CCH3−O∣∣C−CH∣CH3−CH2−CH3DCH3−CH2−O∣∣C−CH∣CH3−CH3Unknown compound (A) C5H10O gives positive test with 2,4-DNP but negative test with Tollen's reagent . It also give yellow precipitate with I2/NaOH. (A) is :
A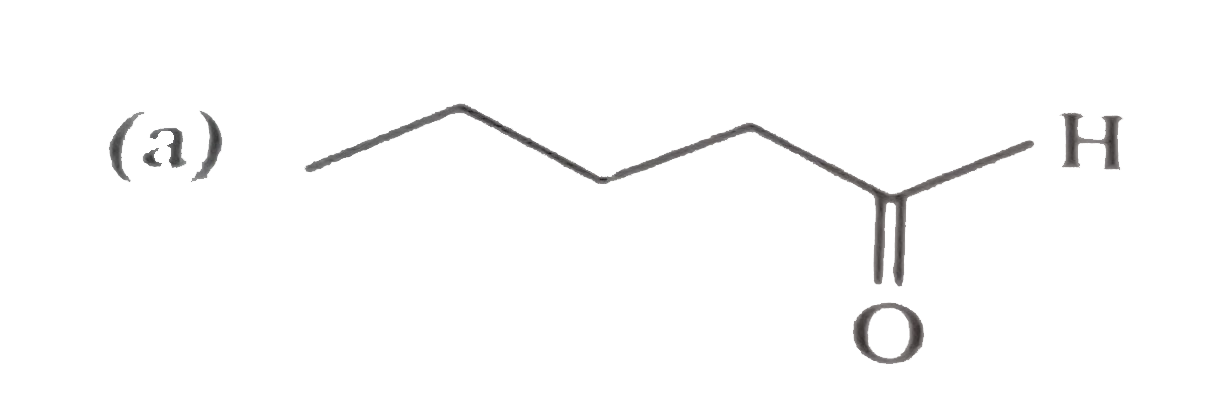 B
B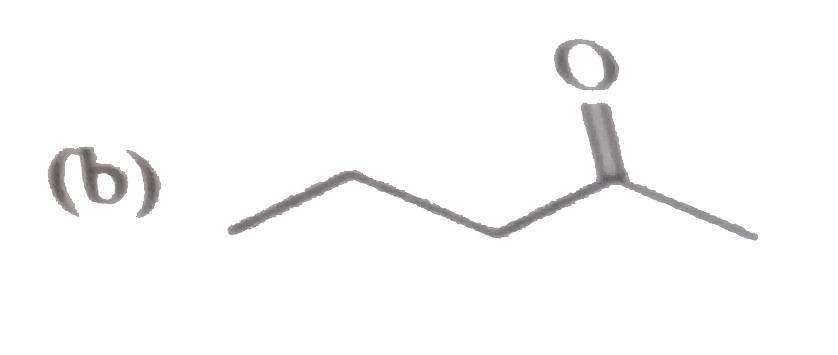 C
C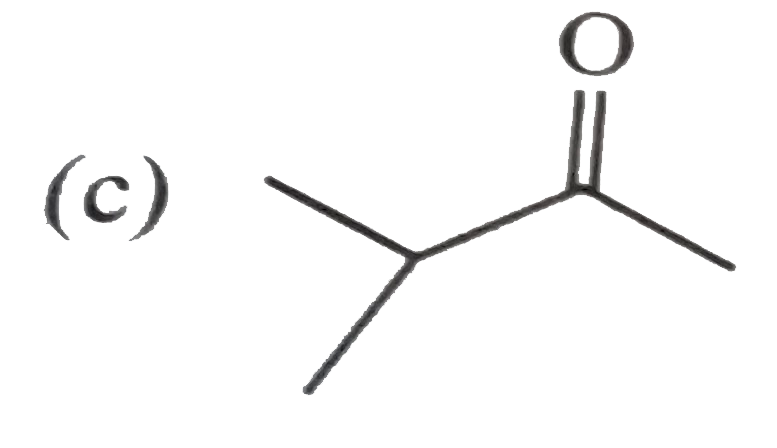 D
D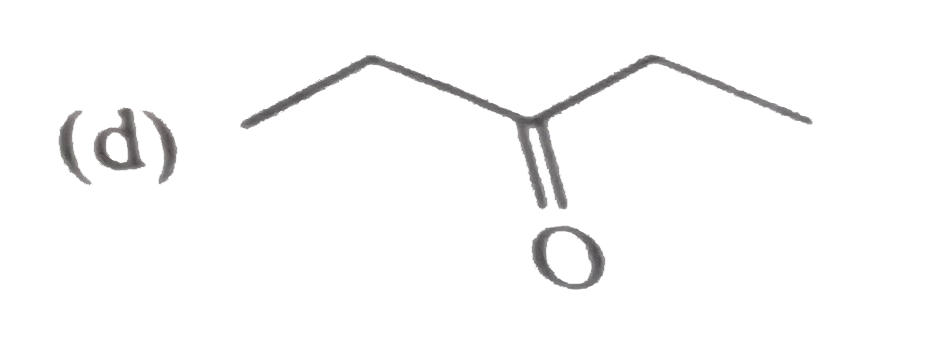
Unknown compound (A) C5H10O gives positive test with 2,4-DNP but negative test with Tollen's reagent . It also give yellow precipitate with I2/NaOH. (A) is :
View SolutionA organic compound with the molecular formula C3H6O does not respond positively to the silver mirror test with Tollens reagent but produces on oxmime. The compound is
View SolutionA compound [A] gives positive iodoform test in 5 minutes. When 6.0 g of [A] is treated with sodium metal, 1120 mL of hydrogen gas is evolved at NTP. It is assumed that [A] contains one atom of oxygen per molecule. Further when [A] reacts with PBr3, a compound [B] is formed which on reacting with benzene in the presence of anhydrous AlCl3 gives a compound [C]. the compound [C] is a well known industrial compound and is used in the commercial preparation of phenol.
The molar mass of compound [A] is :
View SolutionA compound [A] gives positive iodoform test in 5 minutes. When 6.0 g of [A] is treated with sodium metal, 1120 mL of hydrogen gas is evolved at NTP. It is assumed that [A] contains one atom of oxygen per molecule. Further when [A] reacts with PBr3, a compound [B] is formed which on reacting with benzene in the presence of anhydrous AlCl3 gives a compound [C]. the compound [C] is a well known industrial compound and is used in the commercial preparation of phenol.
The compound [C] is
View SolutionA compound [A] gives positive iodoform test in 5 minutes. When 6.0 g of [A] is treated with sodium metal, 1120 mL of hydrogen gas is evolved at NTP. It is assumed that [A] contains one atom of oxygen per molecule. Further when [A] reacts with PBr3, a compound [B] is formed which on reacting with benzene in the presence of anhydrous AlCl3 gives a compound [C]. the compound [C] is a well known industrial compound and is used in the commercial preparation of phenol.
The compound [A] is a
View Solution

