The correct sequence in decreasing order of the precentage of nitrogen in the given compounds is
A
Urea>Ammonium chloride>ammoniumnitrate>Ammonium nitrite
B
Urea>Ammonium nitrate>Ammonium nitrate >Ammonium chloride
C
Urea>Ammonium nitrate >Ammonium nitrate >Ammonium chloride
D
Urea>Ammonium nitrite >Ammonium chloride >ammonium nitrate
Text Solution
The correct Answer is:C
Correct sequence is
NH2CONH2>NH4NO2>NH4NO2>NH4Cl
|
Similar Questions
Knowledge Check
The correct decreasing order for the acidic strength of oxides of nitrogen is
AN2O5>N2O3>N2O4>NO>N2OBNO>N2O>N2O3>N2O4>N2O5CN2O>NO>N2O3>N2O4>N2O5DN2O5>N2O4>N2O3>NO>N2OThe correct sequence which shows decreasing order of the ionic radii of the element is
AAl3+>Mg2+>Na+>F>O2−BNa+>Mg2+>Al3+>O2−>F−CNa+>F−>Mg2+>O2−>Al3+DO2−>F−>Na+>Mg2+>Al3+The correct sequence which shown decreasing order of the ionic radii of the elements is
AAl3+>Mg2+>Na+>F−>O2−BNa+>Mg2+>AI3+>O2−>F−CNa+>F−>Mg2+>O2−>AI3+DO2−>F−>Na+>Mg2+>AI3+
The correct decreasing order of densities of the following compounds is :
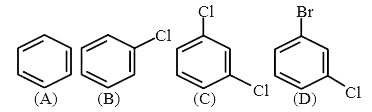
View SolutionThe correct sequence which shows decreasing order of the ionic radii of the element is
View SolutionThe correct sequence of elements in decreasing order first ionisation energy is
View SolutionThe correct sequence which shows decreasing order of the ionic radii of the elements is
View SolutionThe correct sequence of elements in the decreasing order of first ionisation energy is
View Solution

