The most stabl enol tautomer of MeCOCH2CO2Et is
CH2=C(OH)CH2CO2Et
MeC(OH)=CHCO2Et
MeCOCH=C(OH)OEt
CH2=C(OH)CH=C(OH)Et
The correct Answer is:B
To determine the most stable enol tautomer of the compound MeCOCH2CO2Et (ethyl acetoacetate), we will follow these steps:
Step 1: Identify the Keto Form
The given compound is ethyl acetoacetate, which can be represented as:
\( \text{CH}_3\text{C(=O)CH}_2\text{CO}_2\text{Et} \)
This is the keto form of the compound.
Step 2: Draw the Enol Form
The enol form is obtained by the deprotonation of the alpha hydrogen (the hydrogen on the carbon adjacent to the carbonyl group) and the formation of a double bond between the alpha carbon and the carbonyl carbon. The enol form can be represented as:
\( \text{CH}_3\text{C(=C(OH))CH}_2\text{CO}_2\text{Et} \)
Step 3: Analyze the Stability of the Enol Form
The stability of the enol form can be influenced by several factors:
- Hydrogen Bonding: The enol can form intramolecular hydrogen bonds, which can stabilize the structure.
- Resonance: The enol can exhibit resonance stabilization due to the presence of the hydroxyl group.
Step 4: Compare the Stability of Keto and Enol Forms
In general, keto forms are more stable than enol forms due to the presence of a carbonyl group. However, in some cases, the enol form can be stabilized by hydrogen bonding or resonance.
Step 5: Conclusion
For ethyl acetoacetate, the enol tautomer is stabilized by intramolecular hydrogen bonding and resonance. Thus, the most stable enol tautomer of MeCOCH2CO2Et is:
\( \text{CH}_3\text{C(=C(OH))CH}_2\text{CO}_2\text{Et} \)
The given compound is ethyl acetoacetate, which can be represented as:
\( \text{CH}_3\text{C(=O)CH}_2\text{CO}_2\text{Et} \)
This is the keto form of the compound.
The enol form is obtained by the deprotonation of the alpha hydrogen (the hydrogen on the carbon adjacent to the carbonyl group) and the formation of a double bond between the alpha carbon and the carbonyl carbon. The enol form can be represented as:
\( \text{CH}_3\text{C(=C(OH))CH}_2\text{CO}_2\text{Et} \)
The stability of the enol form can be influenced by several factors:
- Hydrogen Bonding: The enol can form intramolecular hydrogen bonds, which can stabilize the structure.
- Resonance: The enol can exhibit resonance stabilization due to the presence of the hydroxyl group.
In general, keto forms are more stable than enol forms due to the presence of a carbonyl group. However, in some cases, the enol form can be stabilized by hydrogen bonding or resonance.
For ethyl acetoacetate, the enol tautomer is stabilized by intramolecular hydrogen bonding and resonance. Thus, the most stable enol tautomer of MeCOCH2CO2Et is:
\( \text{CH}_3\text{C(=C(OH))CH}_2\text{CO}_2\text{Et} \)
Similar Questions
Knowledge Check
Most stable tautomers of
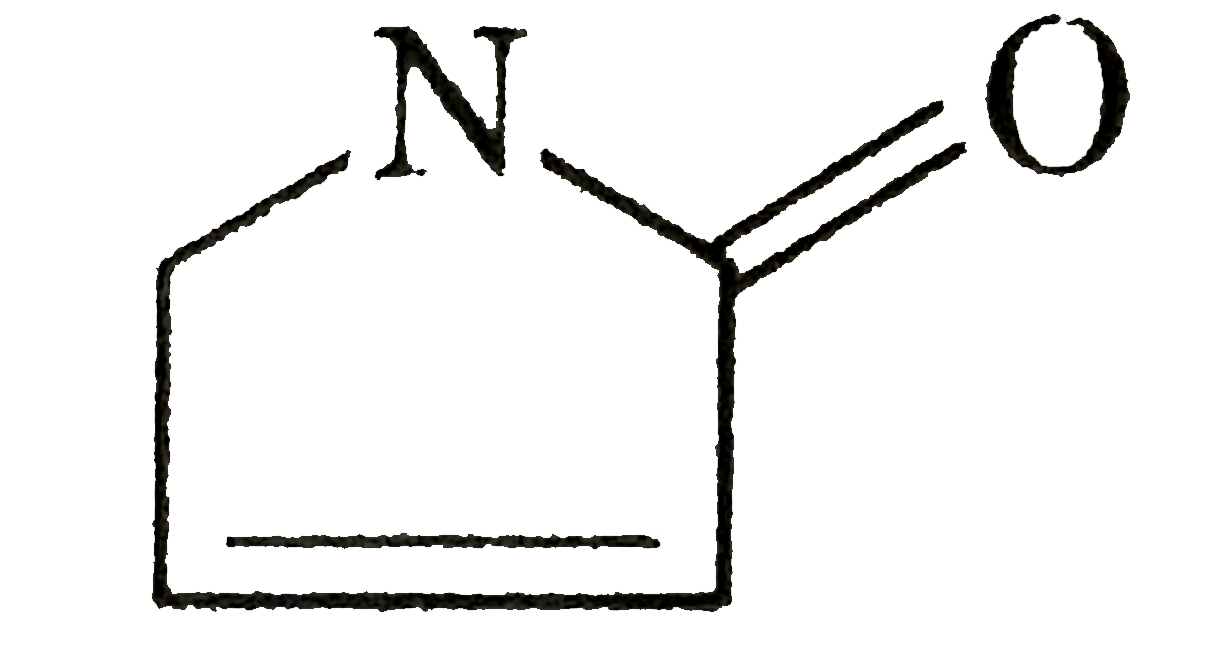 is :A
is :A B
B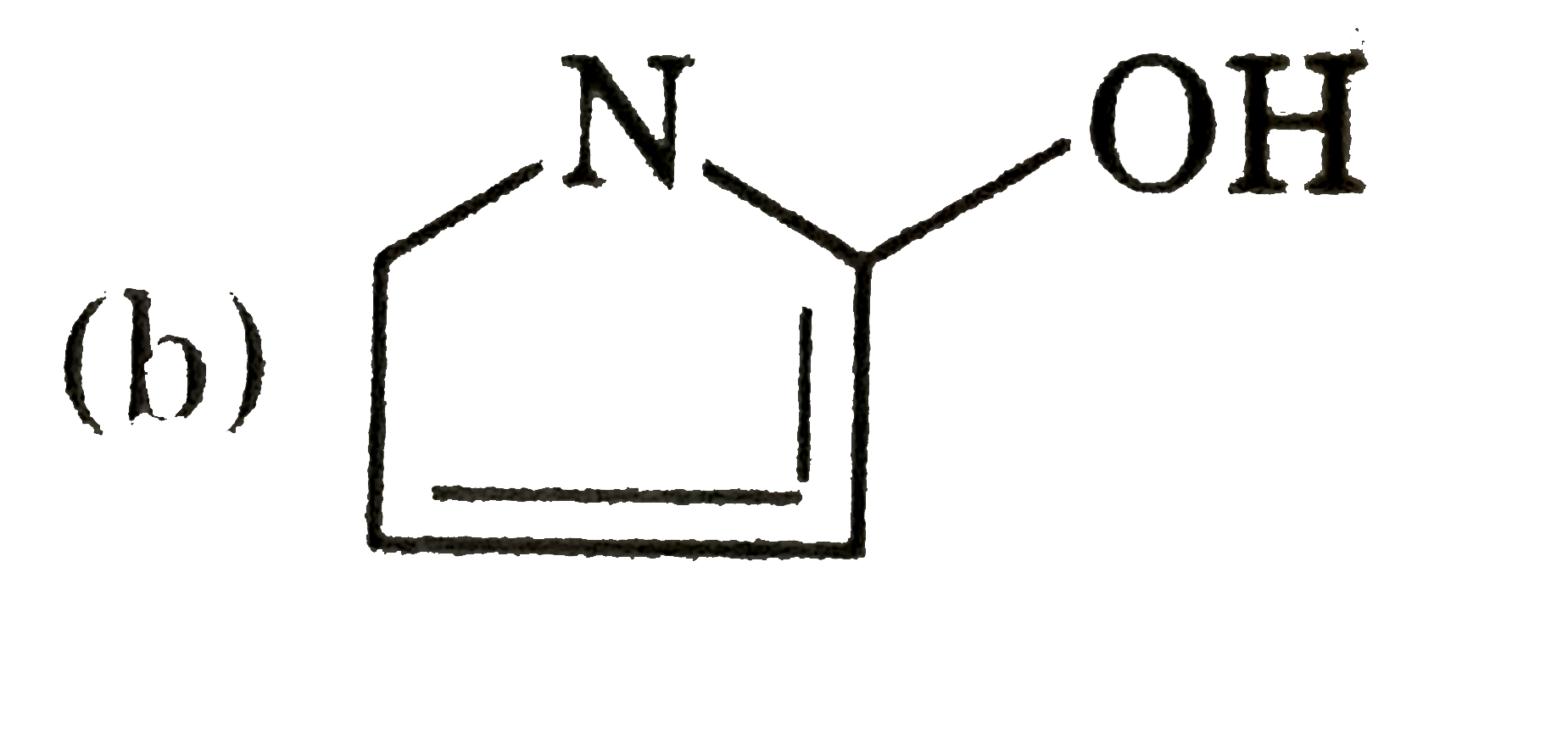 C
C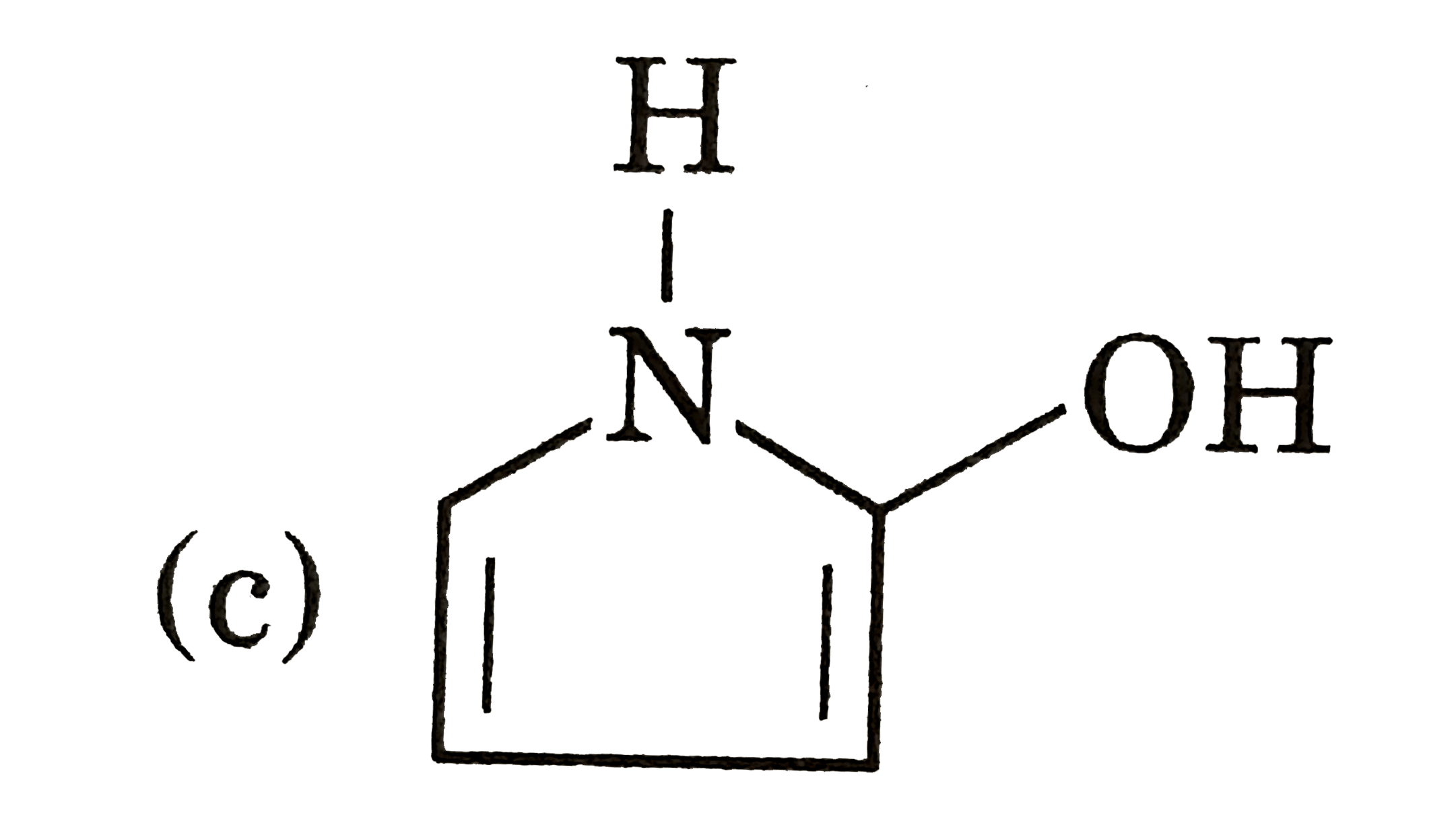 D
D
The most contributing tautomeric enol form of MeCOCH2CO2 Et is:
AH2C=C(OH)CH2CO2EtBMeC(OH)=CHCO2EtCMeCOCH=C(OH)OetDH2C=C(OH)CH=C(OH)EtThe most stabl enol tautomer of MeCOCH2CO2Et is
ACH2=C(OH)CH2CO2EtBMeC(OH)=CHCO2EtCMeCOCH=C(OH)OEtDCH2=C(OH)CH=C(OH)Et
The structrue of the enol form of
CH3−CO−CH2−CO−CH3 with intermolecular hydrogen bonding is…..
View SolutionThe most contributing tautomeric enol form of MecCOCH2CO2Et is
View SolutionThe most stable oxyacid of chlorine is
View SolutionThe most stable among the following is :
View SolutionThe most stable conformer of 2, 4-hexadience is
View Solution
