Amongst the following compounds, identify which are insoluble, partially soluble and highly soluble in water
(v) chloroform
To determine the solubility of chloroform (CHCl3) in water, we can follow these steps:
Step 1: Identify the nature of chloroform
Chloroform (CHCl3) is a molecular compound. It consists of one carbon atom, one hydrogen atom, and three chlorine atoms.
Step 2: Analyze the polarity of chloroform
To understand its solubility, we need to analyze the polarity of chloroform. The electronegativity difference between hydrogen (H) and chlorine (Cl) is not significant enough to create a highly polar molecule. Although chlorine is more electronegative than hydrogen, the overall molecular structure of chloroform is relatively non-polar due to the symmetrical arrangement of the chlorine atoms around the carbon atom.
Step 3: Compare with water
Water (H2O) is a highly polar solvent due to the significant difference in electronegativity between hydrogen and oxygen. The "like dissolves like" principle states that polar solvents dissolve polar solutes, and non-polar solvents dissolve non-polar solutes.
Step 4: Determine solubility
Since chloroform is non-polar and water is polar, they do not mix well. Therefore, chloroform is insoluble in water.
Conclusion
Chloroform (CHCl3) is classified as insoluble in water.
---
Chloroform (CHCl3) is a molecular compound. It consists of one carbon atom, one hydrogen atom, and three chlorine atoms.
To understand its solubility, we need to analyze the polarity of chloroform. The electronegativity difference between hydrogen (H) and chlorine (Cl) is not significant enough to create a highly polar molecule. Although chlorine is more electronegative than hydrogen, the overall molecular structure of chloroform is relatively non-polar due to the symmetrical arrangement of the chlorine atoms around the carbon atom.
Water (H2O) is a highly polar solvent due to the significant difference in electronegativity between hydrogen and oxygen. The "like dissolves like" principle states that polar solvents dissolve polar solutes, and non-polar solvents dissolve non-polar solutes.
Since chloroform is non-polar and water is polar, they do not mix well. Therefore, chloroform is insoluble in water.
Chloroform (CHCl3) is classified as insoluble in water.
Similar Questions
Knowledge Check
Amongst the following compound which is/are soluble in aqueous NaOH?
A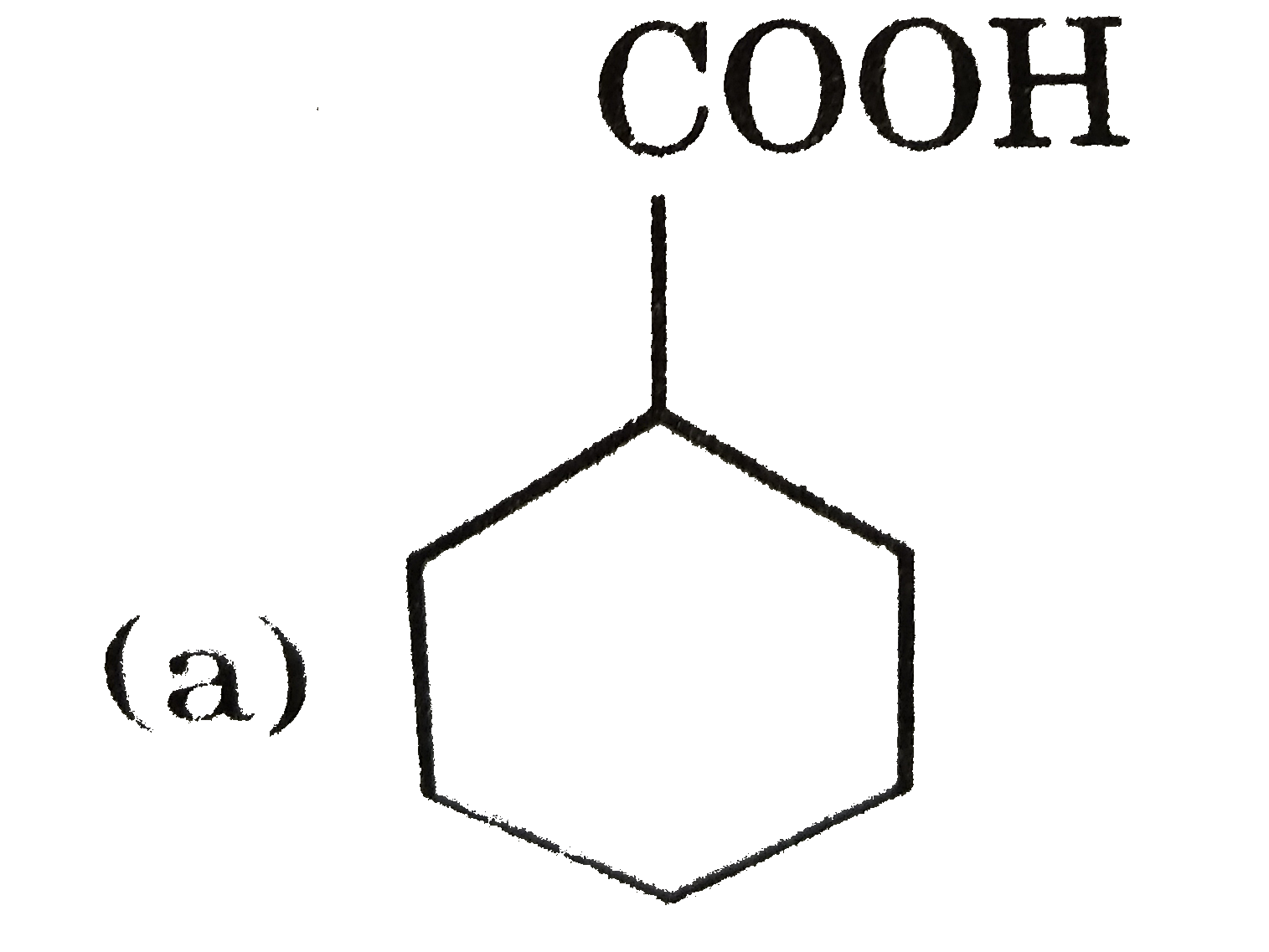 B
B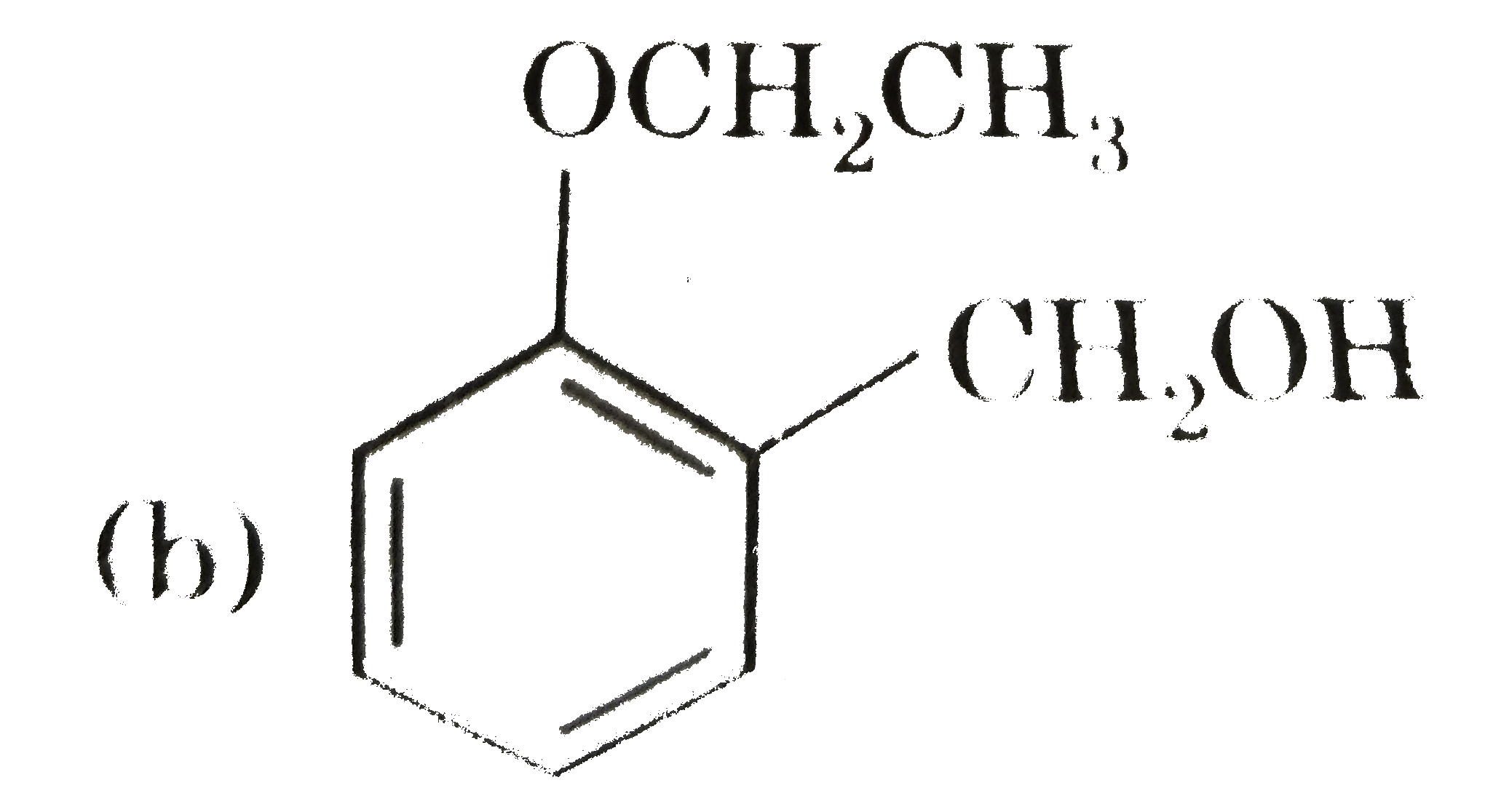 C
C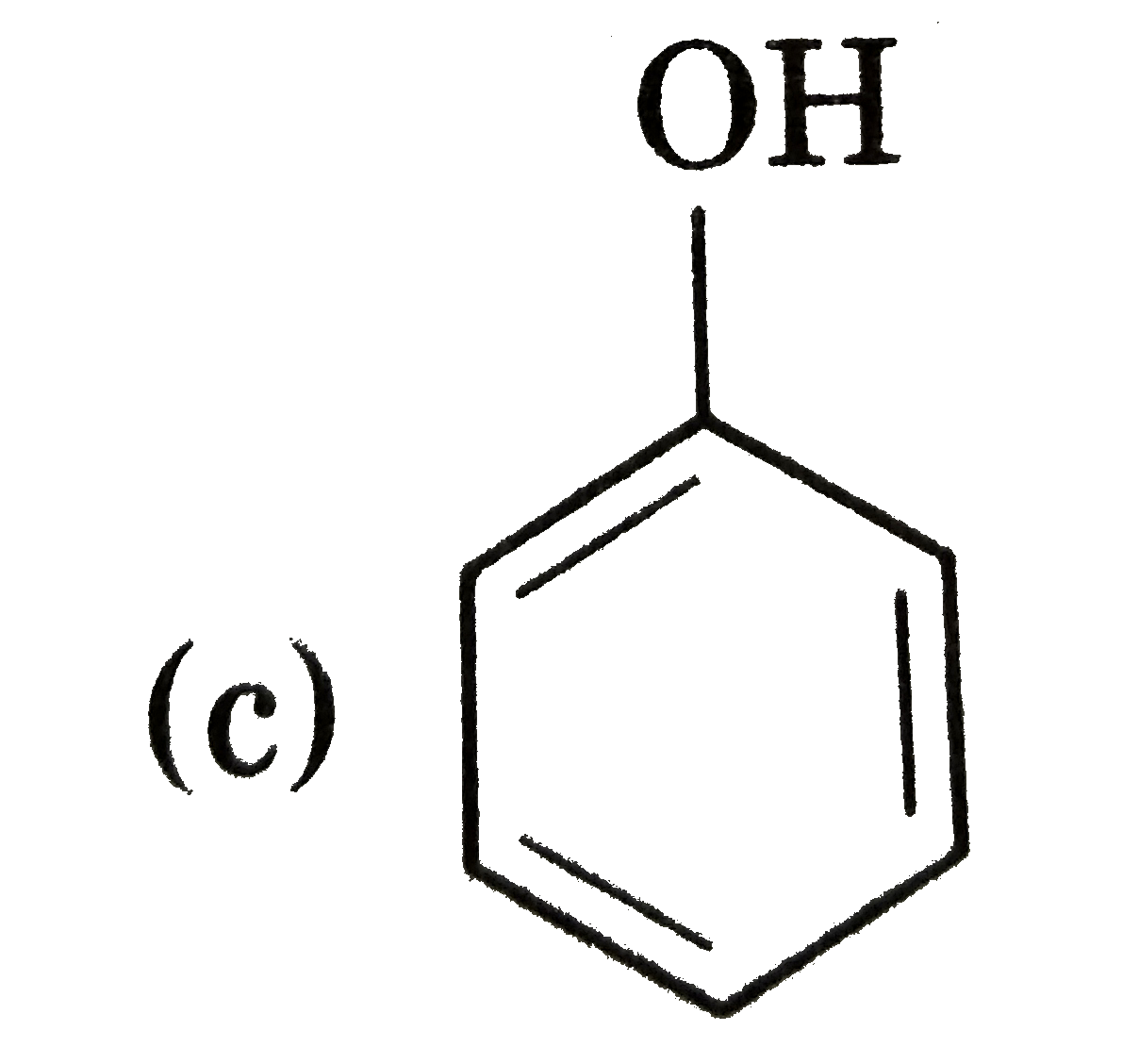 D
D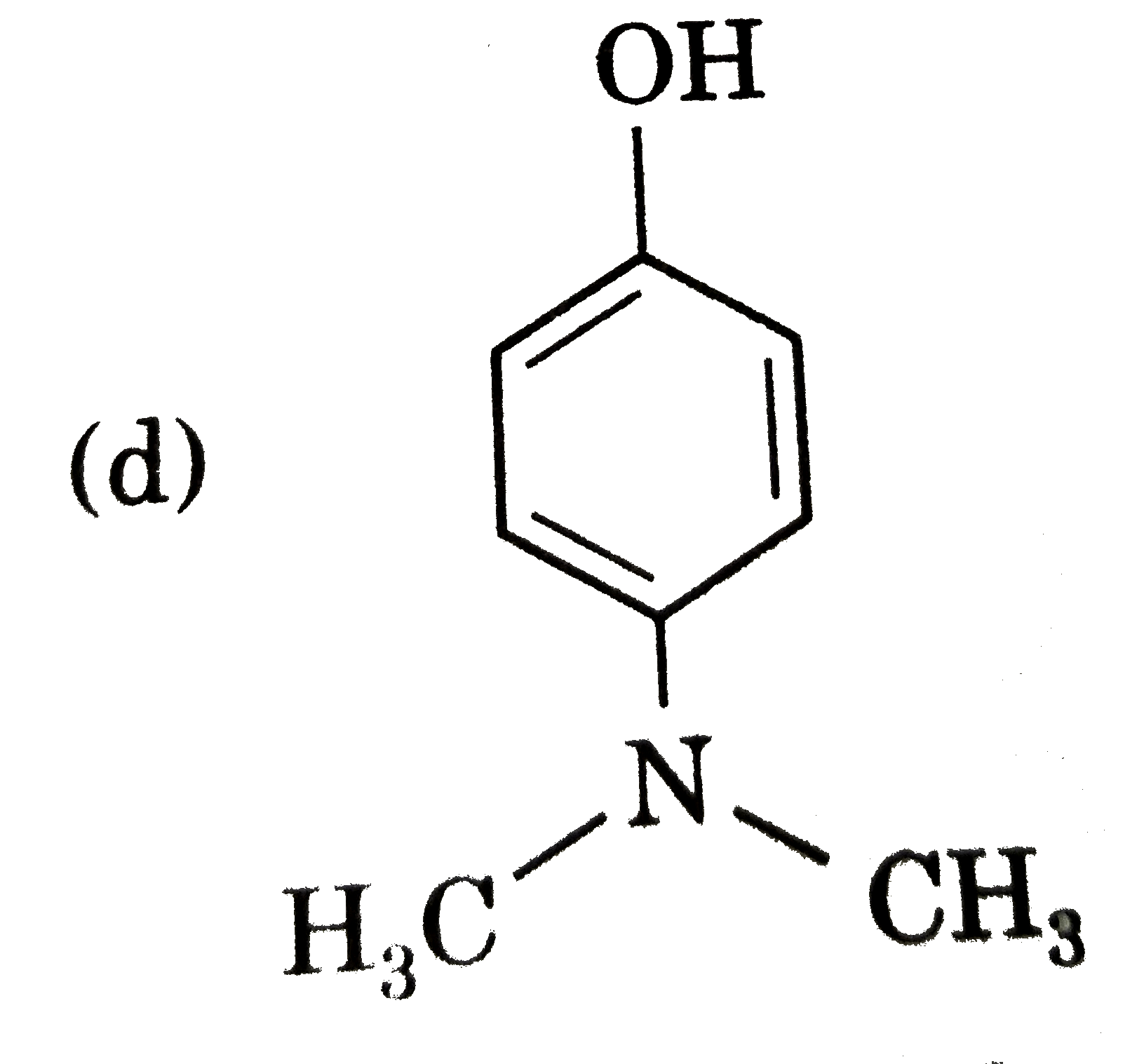
Identify the pair of water soluble bases.
Acopper oxide and potassium oxideBcopper oxide and calcium oxideCmagnesium oxide and potassium oxideDaluminium oxide and magnesium oxide
Amongst the following compounds, identify which are insoluble, partially soluble and highly soluble in water
(ii) toluene
View SolutionAmongst the following compounds, identify which are insoluble, partially soluble and highly soluble in water
(iv) ethylene glycol
View SolutionAmongst the following compounds, identify which are insoluble, partially soluble and highly soluble in water
(iv) pentanol
View SolutionAmongst the following compounds, identify which are insoluble, partially soluble and highly soluble in water ?
(i) phenol (ii) toluene (iii) formic acid (iv) ethylene glycol (v) chloroform (vi) pentanol.
View SolutionAmong the following compounds, identify whiCHM are insoluble, partially soluble, and highly soluble in water ?
a. Phenol b. Toluene
c. Formic acid d. Ethylene glycol
e. CHMloroform f. Pentanol
View SolutionWhy is ammonia highly soluble in water ?
View Solution
