For the decomposition of azoisopropane to hexane and nitrogen at 54
K, the following data are obtained.
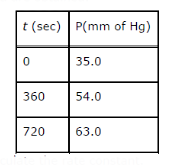
Calculate the rare constant.
Video Solution
Text Solution
(CH3)2CH N=N CH(CH3)2(g)→N2(g)+C6H16(g)
Initial pressure P000After time tP0−ppp
Total pressure after time t(Pt)=(P0−p)+p+p=P0+p or p=Pt−P0
a∝P0 and (a−x)∝P0−p or substituting the value of p,
a−x∝P0−(Pt−P0),i.e.,(a−x)∝P0−Pt
As decomposition of azoisopropane is a first order reaction,
k=2.303tlogaa−x=2.303tlogP02P0−Pt
When t=360 sec,k=2.303360 slog3502×35.0×54.0=2.303360 slog3516=2.303360 s(0.3400)=2.175×10−3s−1
When t=720 sec,k=2.303720 slog35.02×35.0−63.0=2.303720 slog5=2.303720(0.6990)=2.235×10−3s−1
∴ Average value of k=2.175+2.2352×10−3s−3=2.20×10−3s−1.
|
Similar Questions
Knowledge Check
For the decomposition of a compound AB at 600 K, the following data were obtained
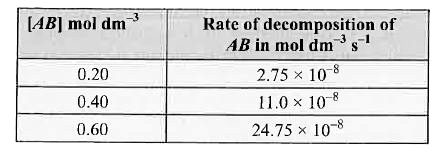
The order for the decomposition of AB isA0B1C2D1.5
For the first order thermal decomposition reaction, following data was obtained:

Calculate the rate constant.
View SolutionFor the decomposition of a compount AB at 600 K, the following data were obtained.

Find the order for the decomposition of AB.
View SolutionCalculate TP from the following data :
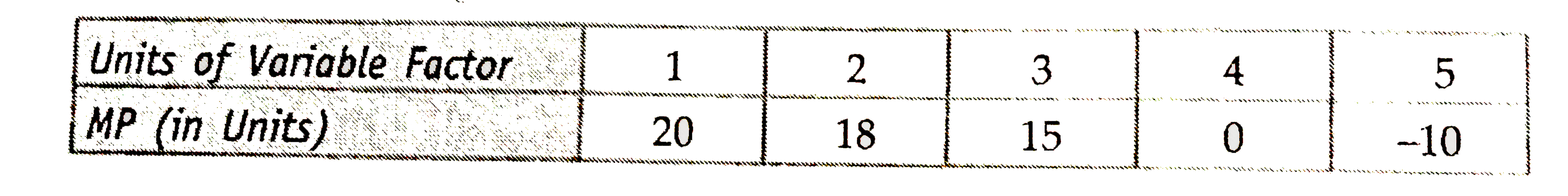
View SolutionCalculate the mean of the following data
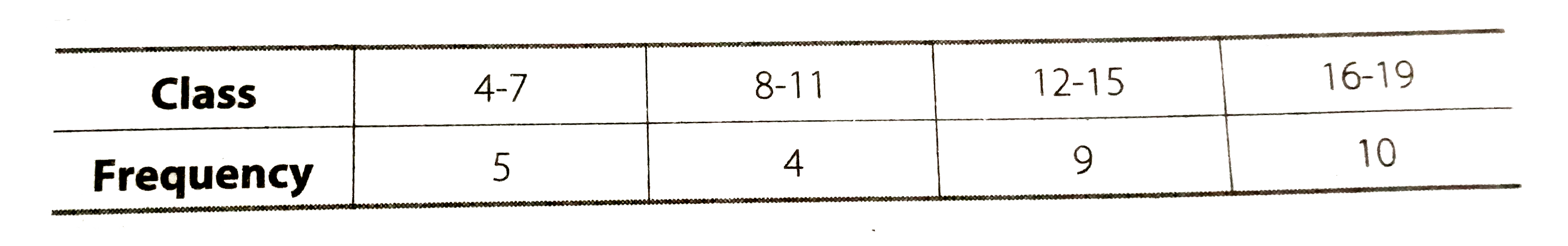
View SolutionCalculate the mean of the following data:

View Solutionform the gaseous reaction
2A+B2→2AB, the following rate data were obtained at 300K.
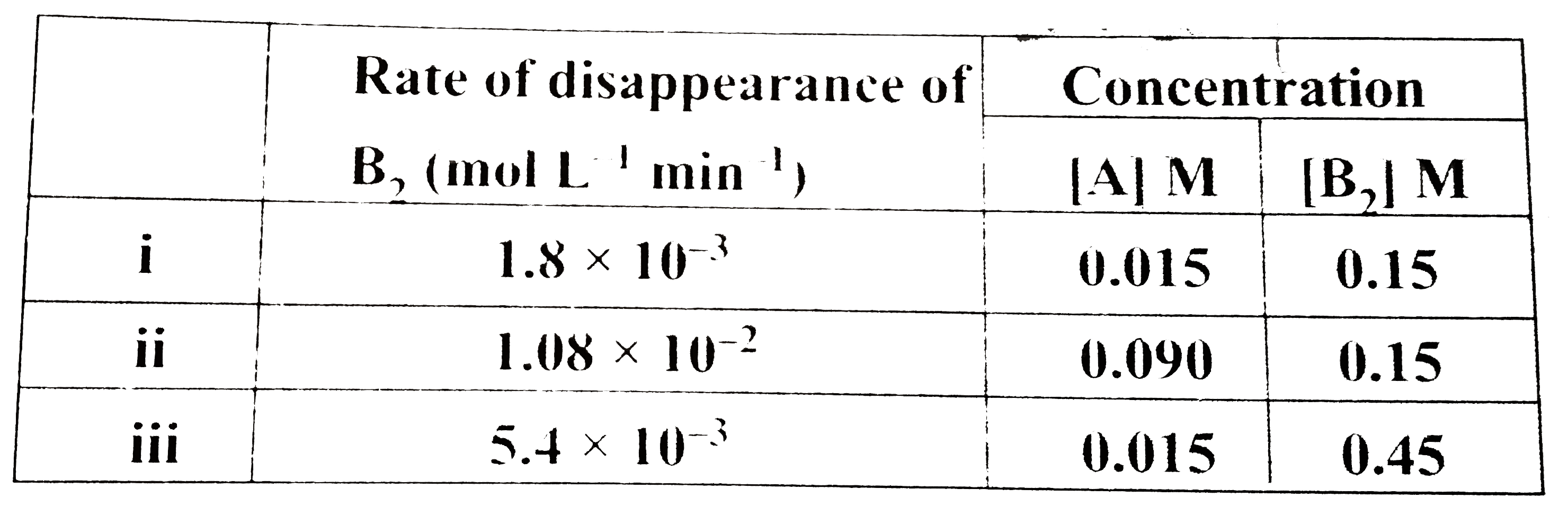
Calculate the rate constant for the reaction and the rate of formation of AB when [A] is 0.02 and [B2] is 0.04molL−1 at 300K.
View SolutionCalculate Sales from the following data :

View Solution


