Sulphuric acid is a dehydrating agent. Give one example.
Step-by-Step Solution:
1. Understanding Sulfuric Acid as a Dehydrating Agent:
- Sulfuric acid (H₂SO₄) is known for its strong dehydrating properties. A dehydrating agent is a substance that removes water from another substance.
2. Identifying an Example:
- One common example of sulfuric acid acting as a dehydrating agent is its reaction with sugar (sucrose), which has the chemical formula C₁₂H₂₂O₁₁.
3. The Reaction:
- When concentrated sulfuric acid is added to sugar, it removes the water molecules from the sugar. The reaction can be represented as follows:
C12H22O11+H2SO4→C+H2O
- In this reaction, the sulfuric acid dehydrates the sugar, resulting in the formation of carbon (C) and water (H₂O).
4. Observing the Result:
- The process is often referred to as the "charring of sugar," where the sugar turns black due to the formation of carbon as the water is removed.
5. Conclusion:
- Therefore, an example of sulfuric acid acting as a dehydrating agent is its reaction with sugar, leading to the formation of carbon and water.
---
- Sulfuric acid (H₂SO₄) is known for its strong dehydrating properties. A dehydrating agent is a substance that removes water from another substance.
- One common example of sulfuric acid acting as a dehydrating agent is its reaction with sugar (sucrose), which has the chemical formula C₁₂H₂₂O₁₁.
- When concentrated sulfuric acid is added to sugar, it removes the water molecules from the sugar. The reaction can be represented as follows:
C12H22O11+H2SO4→C+H2O
- In this reaction, the sulfuric acid dehydrates the sugar, resulting in the formation of carbon (C) and water (H₂O).
- The process is often referred to as the "charring of sugar," where the sugar turns black due to the formation of carbon as the water is removed.
- Therefore, an example of sulfuric acid acting as a dehydrating agent is its reaction with sugar, leading to the formation of carbon and water.
Similar Questions
Knowledge Check
Sulphurous acid is reducing agent due to
Aabsence of hydrogen bondingBpresence of lone pair of electrons of sulphurCabsence of bonding between oxygen atomsDpresence of only three oxygen atomsAssertion : Sugar charcoal is prepared by dehydration of sugar.
Reason : Sulphuric acid is a dehydrating agent.AIf both assertion and reason are true and reason is the correct explanation of assertion.BIf both assertion and reason are true but reason is not the correct explanation of assertion.CIf assertion is true but reason is false.DIf both assertion and reason are false.Which dicarboxylic acid in presence of a dehydrating agent is least reactive for giving an anhydride?
A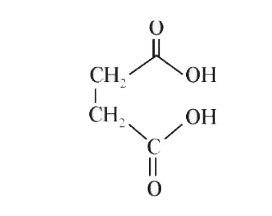 B
B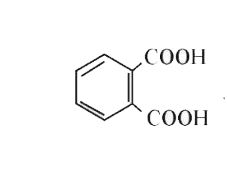 C
C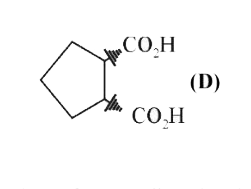 D
D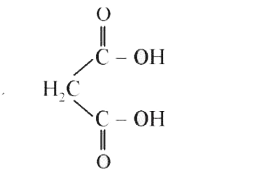
Which dicarboxylic acid in presence of a dehydrating agent is least reactive to give an anhydride ?
View SolutionSulphurous acid is reducing agent due to
View SolutionWhat are artificial sweetening agents ? Give two examples.
View SolutionStatement-1 : Sulphuric acid is less viscous than water due to intermolecular hydrogen bonding.
Statement-2 : Concentrated sulphuric acid is used as dehydrating agent.
View SolutionWhich dicarboxylic acid is presence of dehydrating agent is least reactive to give an anhydride ?
View Solution

