Arrange the following acids in the correct increasing order of their acidic strength,
HCl, HBr, HI, HF
Video Solution
Text Solution
As the size of the atom (X) increases from F to I, the bond length of the H-X bond increases and consequently bond dissociation enthalpy of the H-X bond decreases. In other bonds, bond dissociation enthalpy increases in the ordr : HI<HBr<HCl<HF
|
Similar Questions
Knowledge Check
The correct increasing order of acidic strength is
ACH3OH>H2O>(CH3)2CHOHBH2O>CH3OH>(CH3)2CHOHC(CH3)2CHOH>CH3OH>H2ODH2O>(CH3)2CHOH>CH3OHWhat is the correct increasing order of acidic strength?
APropan-l-ol < 4- methyl phenol < phenolBpropan-l-ol > 4-methyl phenol > phenolCPropan-l-ol < 4-methyl phenol <phenolDPhenol > 4-methyl phenol > Propan-1-olGive the correct increasing order of acid strength.
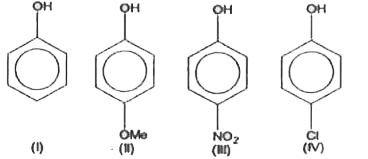 AIII>IV>I>IIBIV>III>I>IICIV>I>II>IIIDI>II>III>IV
AIII>IV>I>IIBIV>III>I>IICIV>I>II>IIIDI>II>III>IV
Which of the following gives the correct increasing order of acidic strength ?
View SolutionWhich of the following gives the correct increasing order of acidic strength ?
View SolutionArrange the following compounds in increasing order of their acidic strength.
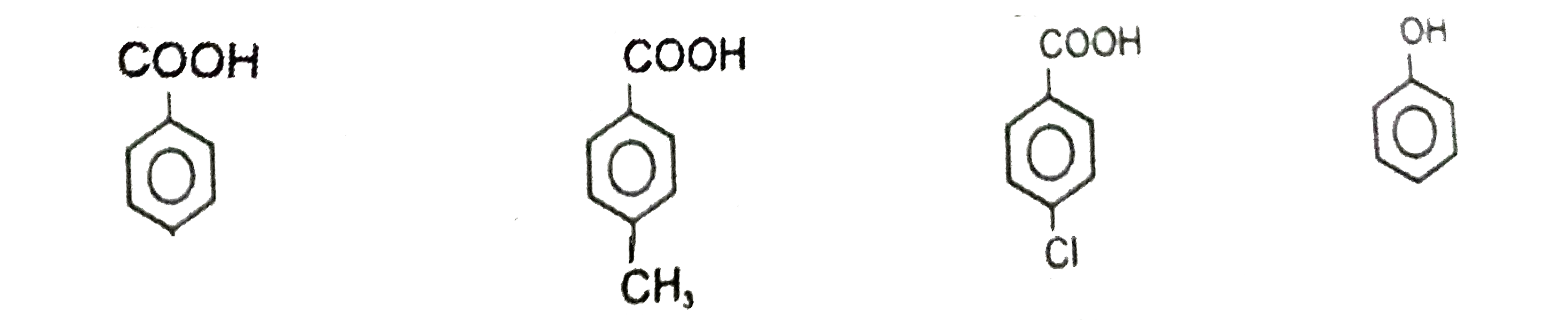
View SolutionWhich of the following gives the correct increasing order of acid strength?
View SolutionArrange the following acid in order of increasing acidity
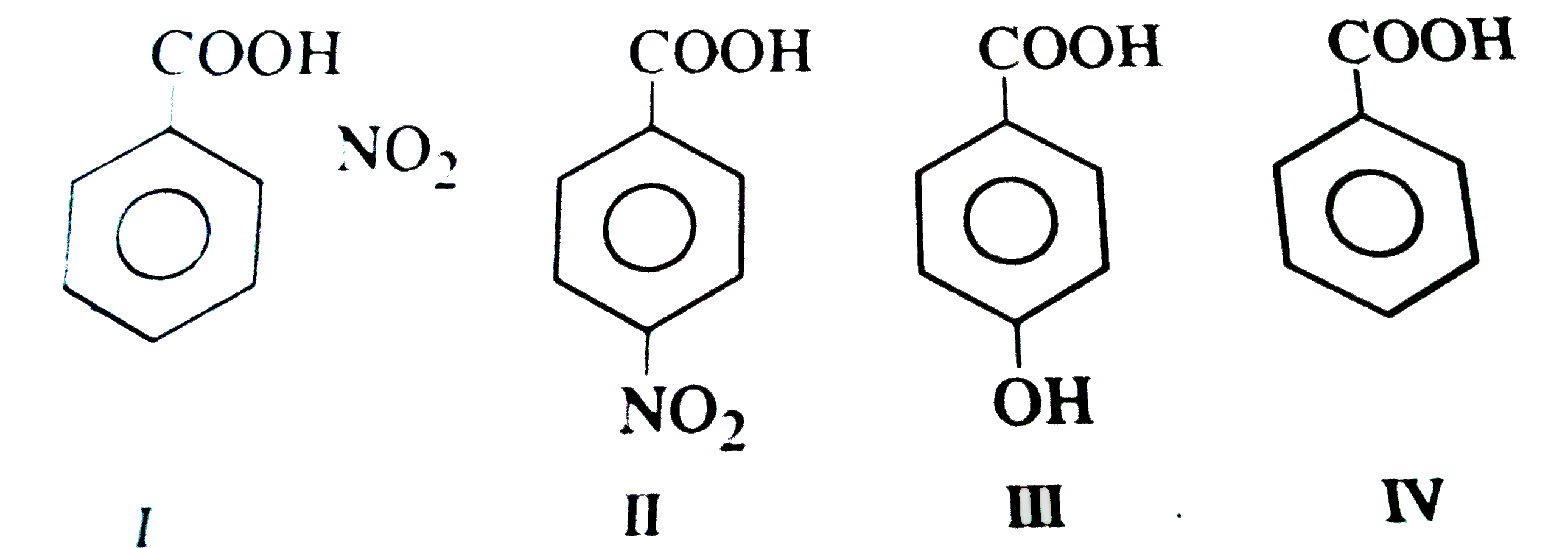
View Solution
