Oxidation number of Cr in the following complex is
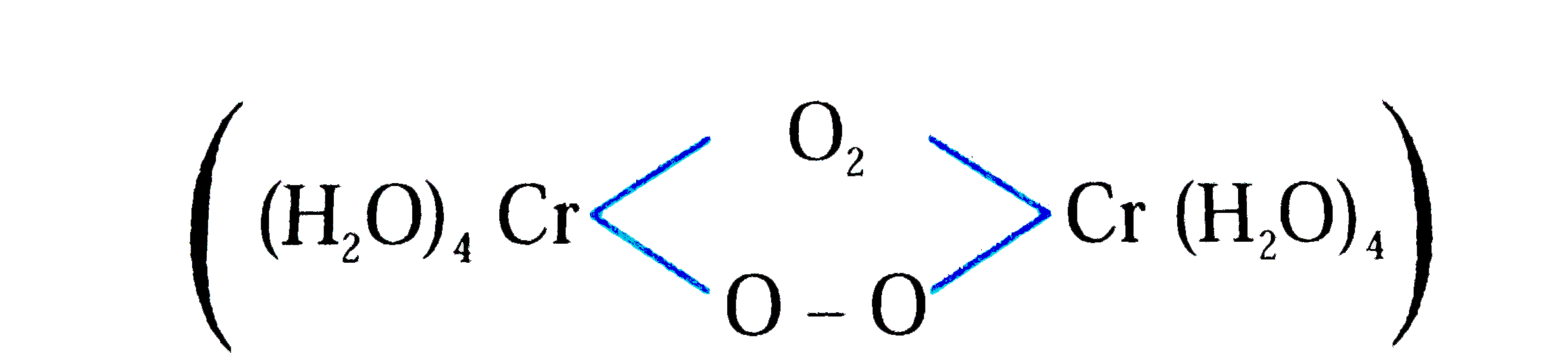
A
2
B
3
C
4
D
5
Video Solution
Text Solution
The correct Answer is:B
2x(for Cr)+12(0)(for H2O)+(−2)(for O-O)peroxideion+(−1)=+3(for O2)superoxideion
or x=+3
|
Similar Questions
Knowledge Check
Oxidation number of Cr in CrO5 is
A+10B+6C+4D+5The oxidation number of Cr is +6 in
AFeCr2O4BKCrO3ClCCrO5D[Cr(OH)_(4)]^(ө)The oxidation number of Co in the complex ion
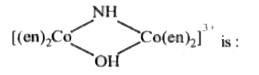 A+2B+3C+4D+6
A+2B+3C+4D+6
The oxidation number of Co in the complex ion
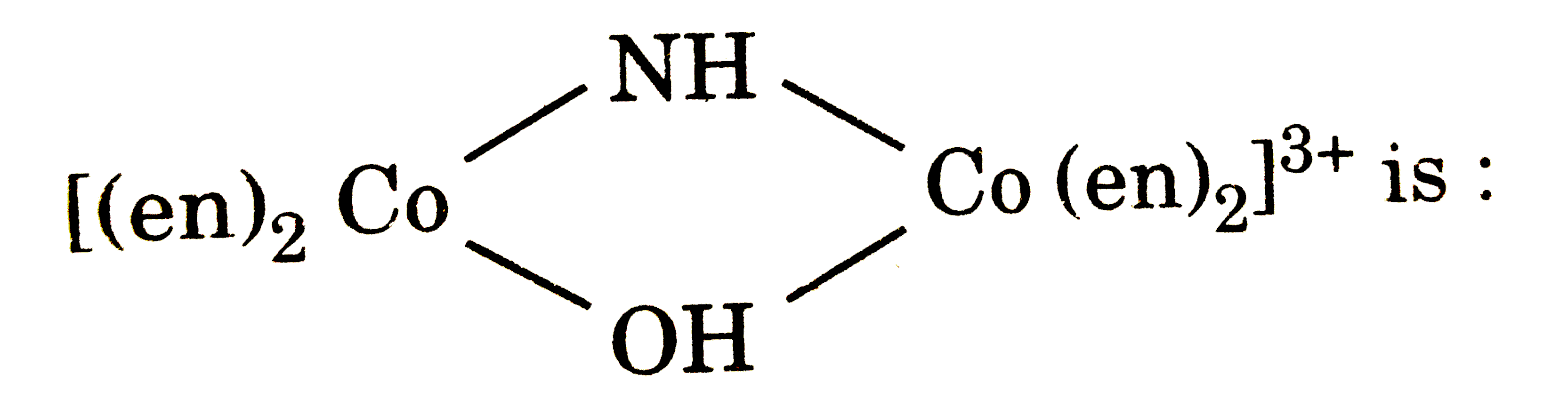
View SolutionThe oxidation number of Co in the complex ion

View SolutionThe oxidation number of Co in the complex ion
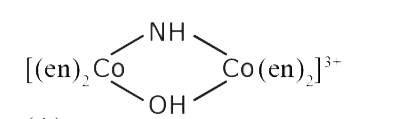
View SolutionThe oxidation number of Co in the complex ion
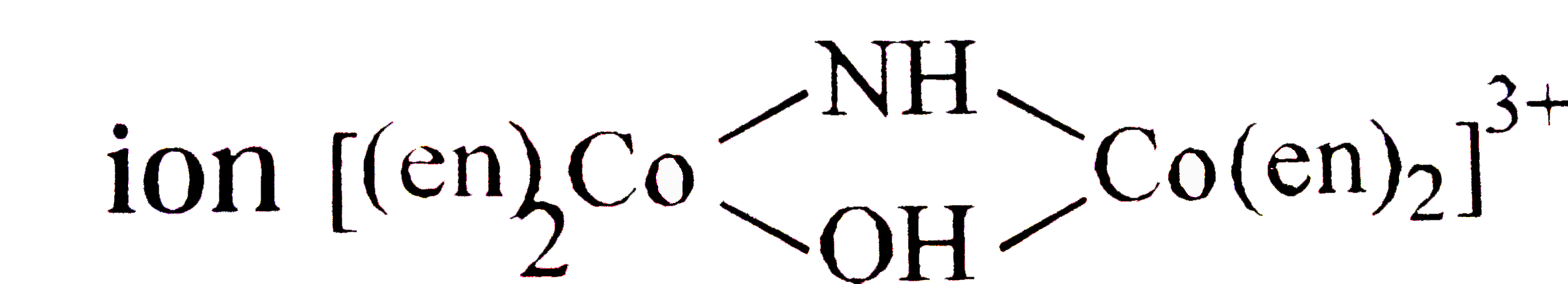
View SolutionThe oxidation number , d-orbital occupation and co-ordination number of cr in the complex
Cis-[Cr(en)2Cl2]Cl are respectively .
View Solution
